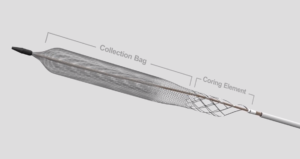
Inari Medical today announced “strongly positive” interim results from the first 250 deep vein thrombosis (DVT) patients enrolled in the CLOUT (ClotTriever outcomes) registry. These latest data showed that the ClotTriever system removed 100% of the blood clots in the majority of DVT patients without the need for thrombolytic drugs in short single-session procedures.
The late-breaking data were presented virtually by principal investigator Robert Beasley (Palm Vascular Center, Miami, USA) at the 2021 New Cardiovascular Horizons (NCVH) conference (1–4 June, New Orleans, USA).
“CLOUT has shown us that by getting between the vessel wall and the thrombus, ClotTriever can remove all of the clot without any injury to the vein or its valves, restoring normal blood flow and valve function,” said Beasley. “For patients with acute, subacute, and chronic clot, ClotTriever has offered long-term relief from the pain associated with DVT and a return to normal life, free from the debilitating symptoms of post-thrombotic syndrome (PTS).”
Use of thrombolytics was completely avoided in all 250 patients across the 24 registry sites and median blood loss was a modest 50ml with a short median thrombectomy procedure time of 28 minutes.
CLOUT is the largest prospective registry ever undertaken of a lytic-free mechanical thrombectomy treatment for DVT. “This latest data readout from the CLOUT registry offers further validation of the frontline role mechanical thrombectomy is playing at a growing number of hospitals in the USA and internationally,” said Bill Hoffman, Inari’s chief executive officer.












