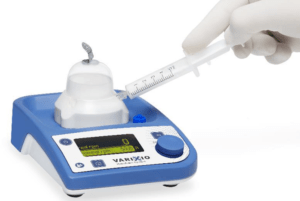
Vascular Barcelona Devices (VB Devices) recently announced that its Varixio Pod Air product received CE mark approval as a Class 1s medical device. Varixio is a novel, patent-protected system that automates the preparation of foam for sclerotherapy of varicose veins.
According to a press release, Varixio is the first device that automates the preparation of foam for sclerotherapy of varicose veins, consistently yielding high-quality, standardised foam, for any sclerosant type and concentration. This will allow to expand the use of foam sclerotherapy as a treatment option to varicose veins of all sizes.
Moreover, Varixio facilitates the work of healthcare providers, adding convenience, reducing preparation time, reducing risks, and leading to higher product performance, and significant indirect cost savings, the press release reads.
“I am very pleased to see this device reach the market and excited about the opportunity it represents to expand sclerotherapy and successfully bring this minimally invasive procedure to even more patients”, said Enric Roche (Hospital Universitari Sagrat Cor, Barcelona, Spain), co-founder of VB Devices.












Buenas tardes, solicito cotización gracias