-
Wed25Mar2026Thu26Mar2026
-
Thu16Apr2026Fri17Apr2026
-
Tue21Apr2026Thu23Apr2026
-
Wed10Jun2026Sat13Jun2026
-
Thu25Jun2026Sat27Jun2026Versailles, France
-
Thu01Oct2026Sat03Oct2026
-
Sat03Oct2026Sun04Oct2026
Latest News
First ACC/AHA acute PE guidelines provide “roadmap” for management
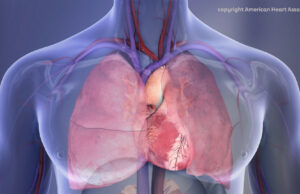
A new severity-based classification system and strong endorsement for multidisciplinary pulmonary embolism response teams (PERTs) feature prominently in recently published multisociety guidelines on the...
Features
Venous News’ top stories of 2025
Which stories were most popular among the venous community in 2025? Read our round-up of the trending articles which appeared across Venous News this year.
1....
“Things are going well”: European Venous Registry secretary reflects on progress...
Speaking to Venous News at this year’s European Society for Vascular Surgery (ESVS) annual meeting (23–26 September, Istanbul, Türkiye), European Venous Registry (EVeR) secretary...
Videos
Steve Elias (Englewood, USA) talks to Venous News about the pivotal trial of high-intensity focused ultrasound (HIFU) using the Sonovein (Theraclion) device during the 2026 American Venous Forum (AVF) in Denver, USA (28 February–4 March). Elias had just presented the...