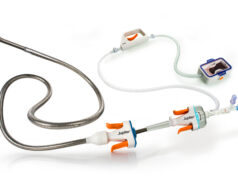
Sapheon announced one year data in a 38-patient clinical study of the VenaSeal Sapheon Closure System, an advanced approach to the treatment of saphenous vein disease based on a proprietary medical adhesive.
All patients were treated without the use of general or tumescent anesthesia or post-procedure compression stockings, and all patients returning for follow up experienced clinical improvement at 1, 3, 6, and 12 months with complete closure rate of over 94% after one year.
“Non-thermal [VenaSeal] adhesive technology for the treatment of saphenous vein incompetence is proving to be safe and durable. At one-year follow-up, the results of this study are quite comparable to methods using thermal ablation,” said Jose I Almeida, study’s chief clinical investigator.
Edward Mackay, St Petersburg, Florida, USA, who also participated in this study, added: “My experience with the VenaSeal System has been very positive based on ease of use and patient tolerance both during the procedure and up to one year post operatively. This has the potential to change the way varicose veins are treated.”
“The VenaSeal Sapheon Closure System was designed to address the more than 1,000,000 new patients each year who undergo invasive and painful surgery or ablation procedures,” said Rod Raabe, chairman and chief medical officer of Sapheon. “We believe that the VenaSeal System is the future for treating venous reflux disease.”
About the VenaSeal Sapheon Closure System
The VenaSeal Sapheon Closure System consists of a proprietary vein sealant and custom delivery system that eliminates the need for painful and time consuming deep tissue injections of tumescent anesthesia. The procedure is performed under ultrasound imaging guidance and requires only local anesthesia at the catheter entry site.