
Venous News talks with Gerard O’Sullivan, FSIR, FEBIR, University College Hospital, Galway, Ireland. This article is supported by BD Interventional.
Disclosures: Dr. Gerard O’Sullivan reports he is a consultant/speaker for BD Interventional, Aspirex, Boston Scientific Corporation, Cook Medical, Marvao Medical, and Optimed. Dr. Gerard O’Sullivan reports that BD Interventional compensated him for his time associated with this article. Dr. Gerard O’Sullivan can be contacted at [email protected].
The venous space continues to evolve in the United States, and many physicians are learning how to navigate through the challenges of the venous anatomy from their international peers. As a well-respected expert, the Galway-based interventional radiologist Dr. Gerard O’Sullivan has substantial experience using venous stents and offers unique insight into the development and design of the Venovo™ Venous Stent System. Venous News highlights below Dr. O’Sullivan’s thoughts on best clinical practices, the significance of the VERNACULAR clinical study, and a case overview demonstrating critical attributes of the Venovo™ Venous Stent.
Understanding potential venous challenges
“Venous is on the upswing at the moment,” O’Sullivan states, but it’s critical to note that physicians new to this space simply cannot apply the same arterial techniques for patient care to treat the deep venous system. The iliac and femoral veins have unique challenges that must be addressed to reduce venous hypertension caused by occlusive disease. Some specific technical issues that may arise in the deep venous system can include large-caliber veins, diffuse and focal lesions, extrinsic compression, post-thrombotic change, and complex curvature.
“The more severe the disease, for instance, CEAP 5 and 6 with ulceration either active or healed, are those that will tend to need extensive work, such as stenting,” suggests O’Sullivan. Physicians should evaluate lesion type, multi-segments of disease, lesions across the inguinal ligament, and if appropriate, when to stent. As an investigator on various venous stent trials and co-principal investigator on the VERNACULAR clinical study for the Venovo™ Venous Stent, Dr. O’Sullivan understands the need behind developing a dedicated venous stent to treat deep vein thrombosis in the iliofemoral veins. “A proven stent with a proven track record is an attractive proposition,” says O’Sullivan. The purpose-built, Venovo™ Venous Stent has a balance between radial strength, compression resistance, and flexibility to address these types of venous challenges.
O’Sullivan further describes, “there are key differences between treating patients with non-thrombotic iliac vein lesions (NIVL) versus those with post-thrombotic syndrome (PTS). Although NIVLs can occur in multiple regions throughout the iliac system, the most common position is where the right common iliac artery compresses the left common iliac vein against the spine. Regardless of position, NIVL lesions tend to be shorter, so you want that stent to land precisely; covering the lesion -and extending slightly above it to accommodate arterial pulsation and mobility; while distally it extends a variable distance inferior to that position. Furthermore, it must achieve an adequate diameter at the NIVL point, which can often involve a quite severe compression,” he affirms. Being one of few nitinol venous stents available in the U.S., O’Sullivan outlines why the Venovo™ Venous Stent delivery system is “easy to use and deploy.” He believes it offers a key benefit of precise placement accuracy and minimal foreshortening.
Shifting focus to PTS lesions, O’Sullivan explains, “PTS patients tend to have a much longer segment of disease, which frequently extends from the upper left common iliac vein, all the way down to the mid-common femoral vein.” He continues, “depending upon the height of the patient, this distance can be anywhere from 18–24 cm, which is where the Venovo™ Venous Stent comes into play. The size offering has a variety of lengths and diameters to choose for very precise patient needs.” Currently, the Venovo™ Venous Stent is the only U.S. venous indicated stent that offers long lengths up to 160 mm with large diameters up to 20 mm. O’Sullivan also mentioned, “when physicians move to the more experienced practices to treat chronic DVTs, particularly patients with CEAP 5 and 6 disease or ulcerations, they’re the ones I think are going to get the most benefit.”
Regarding the possible clinical implications of a stent fracture in the iliofemoral veins, “in my experience, stent fractures are relatively uncommon, except near the inguinal ligament. If two open-cell stents overlap underneath the inguinal ligament, their flexibility decreases. In cases where the patient has severe PTS, which extends into the common femoral vein, or even below, it is preferable to choose a stent that lands well away from the inguinal ligament so that the overlap zone is in the mid-external iliac vein for instance. Often, this means deploying the lowermost stent first,” O’Sullivan states. Physicians worry that stents will crush, displace or fracture, however, O’Sullivan has not observed this occurring with the Venovo™ Venous Stent. The device has been proven safe and effective with zero stent fractures at 12-months.1
Ultimately, by understanding challenges for both NIVL and PTS lesions, the Venovo™ Venous Stent once again brings to light why stent design matters. Its proven performance is a testament to the stent’s careful design together with a balance between strength and flexibility that offers optimal stent placement and lesion coverage with its triaxial delivery system.1
Stent design and selection
Globally, there are a lot of well-established venous stents, but, as O’Sullivan suggests, there are ways of narrowing down the available choices and making a final selection. Above all, balance is key – “I think that venous stents must have a good balance between radial force, flexibility, and conformability, as this affects the overall performance of a stent in the venous anatomy. Stents must be strong enough, in terms of radial force, to be able to combat external compression, and avoid migration, all while being flexible enough to conform to the veins—as opposed to making the vein conform to the stent. This is yet another benefit of the Venovo™ Venous Stent, which has the right balance between radial force and flexibility. These features together with the stent’s flared ends, designed to prevent stent migration, make a well-balanced stent,” O’Sullivan adds.
From experience, he mentions that the use of the Venovo™ Venous Stent has made a difference in his treatment approach for iliofemoral venous disease. “As a result, sizing and placement can be much more precise, which is important for achieving a high level of technical success.” Typically for venous stents, there is a trade-off between radial force and flexibility. “Over time, if the end of the stent lands on a curve, a given stent may straighten and occlude the vein by essentially abolishing that curve. With so many different Venovo™ Venous Stent lengths to choose from, there are various options for precise placement.” Overall, stent placement can affect the technical success and hemodynamics of the vessel.
The importance of evidence
Turning his attention to the data, O’Sullivan highlights the importance of having clinical studies for dedicated venous stents, and how he uses this information for his treatment algorithm and stent selection. In March 2019, the U.S. Food and Drug Administration (FDA) approved of the Venovo™ Venous Stent. The device was evaluated through an Investigational Device Exemption (IDE) VERNACULAR study, a prospective, multi-center, non-randomized, single-arm study for the treatment of symptomatic iliofemoral venous outflow obstruction. One-hundred-seventy participants from 21 investigational sites in the U.S., Europe, and Australia were enrolled in the study. Participants were divided into one of two groups—NIVL and PTS lesions.
Between both cohorts at 12-months, the clinical findings showed an overall weighted primary patency rate of 88.3% (96.9% patency rate in NIVL and an 81.3% patency rate in PTS lesions), displaying a statistically significant difference from the performance goal of 74%. The freedom from target lesion revascularization was 92.6% (n=163) across all the patients followed up to one year. The Venovo™ Venous Stent was deployed successfully to the target lesion, displaying 100% placement accuracy and showed adequate coverage in all cases with zero stent fractures. Moreover, results showed statistically significant improvements in both the VCSS Pain Score and CIVIQ-20 score from baseline to 12-months.
The VERNACULAR results demonstrated a sustained benefit at one year, with both the patency of the stent and the quality of life for patients. “The results so far have exceeded expectations in both the NIVL and PTS populations, and long-term follow up is key for understanding the clinical outcomes,” OSullivan states. Subjects in the VERNACULAR clinical study will be followed for 36 months.
Alongside Dr. O’Sullivan was Dr. Michael Dake (University of Arizona, Tucson, U.S.), principal investigator of the VERNACULAR clinical study, who commented following the FDA-approval of the device, “the unique attributes of the Venovo™ Venous Stent make it particularly well-suited to treat iliofemoral occlusive disease. Most importantly, it is purpose-built for application in veins, and engineered to address the special challenges of venous lesions that are very different than those posed by arterial narrowing.”
Iliofemoral vein stent selection and procedure
Making the stent selection:
Given the chronic nature of disease in iliac segment, there are a handful of stent considerations to evaluate before selection. A durable stent with good radial force and compression resistance is critical in order to withstand the external forces occurring on the vein. In addition, placement accuracy is key, as the stent needs to land precisely at the confluence to avoid jailing off the contralateral vessel. Based on the stent’s design and proven performance, a Venovo™ Venous Stent was selected for this patient.
Patient overview:
- Patient presented with a 12-day history of significant left leg swelling
- CT venography demonstrated an acute left iliofemoral venous thrombosis extending to mid-thigh
- Risk factors included a probable left common iliac vein compression syndrome (May-Thurner) and the oral contraceptive pill
- The risks, benefits, and alternatives of the procedure were discussed in detail with the patient, and informed consent was obtained to proceed
- The patient was brought to the interventional suite, laid prone on the table, and the skin of the left popliteal region was prepped and draped in the usual sterile fashion
- Access was gained to the popliteal vein using ultrasound guidance, and then ascending venography was performed
Venographic findings:
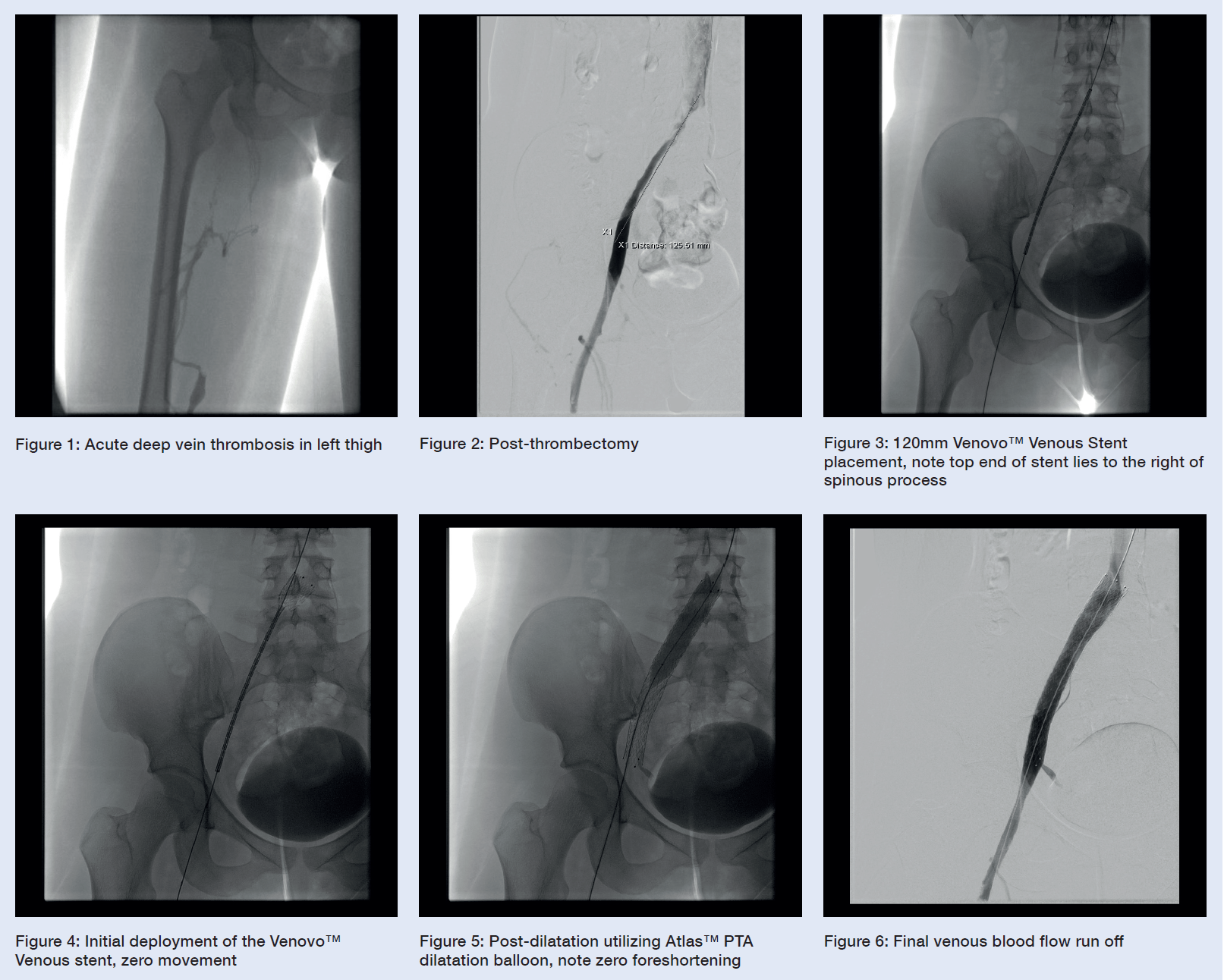
There is occlusive thrombus from the upper popliteal vein with a very well-developed profunda femoris collateral, suggesting that the situation is much more chronic than initially anticipated. Below the level of the chronically occluded left common iliac vein, there is acute thrombus in the left external iliac vein, left common femoral vein, and the profunda femoris vein. (See Figure 1)
Procedure:
- 10F popliteal venous sheath; 5000 units of intravenous heparin were given systemically.
- Using a standard over-the-wire technique, a thrombectomy system was inserted, and free retrograde direction pullback venous thrombectomy was performed. (See Figure 2)
- No thrombolysis was administered.
- Aspiration thrombectomy was performed with an 8-French, 55cm, hockey-stick catheter. A reasonable amount of chronic thrombus was removed, including profunda femoral vein origin thrombus.
- Balloon angioplasty selected, for pre-dilatation of the left common femoral vein, left external iliac vein and left common iliac vein, with a 14mm Atlas™ Gold PTA dilatation balloon, which was extremely painful for the patient, suggesting again, considerable chronicity of their venous disease.
- A single Venovo™ Venous Stent, 14mm diameter, 120mm long stent was deployed, with the proximal end placed in the very distal inferior cava just to the right of the spinous process, and the distal end in the left external iliac vein. (See Figures 3 and 4)
- Post-dilatation completed with balloon angioplasty using an Atlas™ Gold PTA dilatation balloon to maximize wall apposition. (See Figure 5)
- Completion venography reveals rapid in-line flow from a popliteal venous injection up through the scarred femoral vein, with well-developed profunda femoris collaterals, and some collaterals in the obturator region also, through the common femoral vein to the external iliac vein, into the stent, and then into the inferior cava.
- Final image appearances display a satisfactory outcome, given the chronic nature of the femoral venous thrombus. The stent precision is highlighted below and is very visible and very accurate. (See Figure 6)
Follow up protocol:
For post-stenting anticoagulation and imaging, O’Sullivan recommends prescribing low molecular weight heparin for two weeks and then Coumadin or a novel oral anticoagulant (NOAC) for three to six months, depending on the clinical situation. For imaging, he carries out a color doppler ultrasound on day one, a CT venography at six weeks, and clinical review after that. Crucially though, stent failure tends to occur within the first 24 hours, meaning that the use of pneumatic compression boots, thigh-high compression stockings, and early mobilization is critical.






