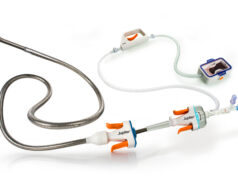
BiO2 Medical has announced that it has received CE mark approval for the Angel Catheter, a nitinol inferior vena cava (IVC) filter, permanently attached to a central venous catheter (CVC) for the use of preventing pulmonary embolism in critically ill patients with venous thromboembolism for whom anticoagulation therapy is temporarily contraindicated.
According to a company release, the Angel device is the first IVC filter with a prophylactic use indication, and allows attending physicians the ability to place an IVC filter bedside in the intensive care unit, in a procedure resembling a routine CVC placement procedure.
“Obtaining the CE clearance for commercialisation of the Angel catheter in Europe is a significant step in our goal to provide an alternative for critically ill patients at high risk of pulmonary embolism. It also validates a long and complex process of extensive testing for the use of our device as a prophylaxis against pulmonary embolism,” said Luis F Angel, chief medical officer, BiO2 Medical, and inventor of the Angel catheter.