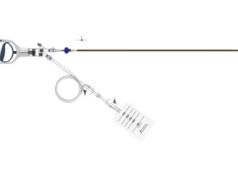
ArtVentive Medical has announced enrolment in the ongoing OCCLUDE post-market surveillance study. This study aims to further the use of the EOS endoluminal occlusion system for the treatment of venous and arterial cases in which precise placement and immediate, efficient vessel embolisation are paramount.
The first study case was performed at The University Hospital, Leuven, Belgium, where Geert Maleux successfully treated a patient with the device. Maleux, a professor in the Department of Radiology, states, “The ArtVentive EOS device enhances our ability to treat challenging embolisation cases. It offers immediate and complete occlusion. The operator can have confidence that the target vessel is occluded. The ArtVentive EOS technology allows for shorter procedure times and potentially reduced radiation exposure for both the physician and patient.”