Bryan Kay
VenoValve: Analysis finds similar improvement among both primary and thrombotic deep...
A new subanalysis of the SAVVE (Surgical antireflux venous valve endoprosthesis) trial found that there was no difference in the level of improvement in...
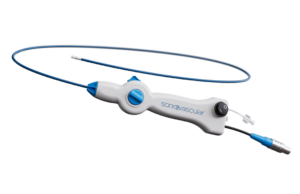
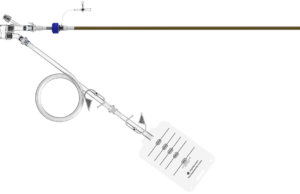
Novel thrombectomy system for VTE used successfully in first tranche of...
SonoVascular has announced the successful completion of an initial set of eight deep vein thrombosis (DVT) cases using its SonoThrombectomy system as part of...
PMA application submitted for VenoValve device
enVVeno Medical today announced it has submitted an application with the U.S. Food and Drug Administration (FDA) seeking approval to market the VenoValve—a surgical...
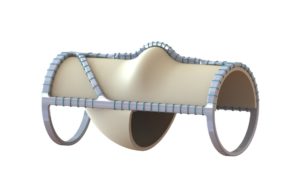
Bioprosthetic venous valve imparts sustained clinical improvement at one year regardless...
A new paper that drills into the impact of a novel bioprosthetic venous valve replacement by CEAP (Clinical, Etiological, Anatomical and Pathophysiological) classification shows...
Pilot study looks at undiagnosed vascular disease in homeless
A pilot study in Canadian capital Ottawa aimed at uncovering the extent of undiagnosed vascular disease among the city’s homeless population detected a subset...
First-in-human study of SonoThrombectomy system completed
SonoVascular has announced the successful initiation of its first-in-human study (FIH) of the company's SonoThrombectomy system, an ultrasound-facilitated, thrombolytic-enhanced platform that utilises multiple mechanisms...
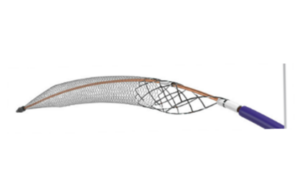
Inari Medical updates ClotTriever XL IFU amid US FDA recall notice
The US Food and Drug Administration (FDA) has issued a Class I recall—the most serious type—for Inari Medical's ClotTriever XL, 30mm device due to...
Eastern Vascular Society set to stage day of service during 2024...
The Eastern Vascular Society (EVS) has incorporated a day of service into its 2024 annual meeting, being held in Charleston, South Carolina (Sept. 19–22).
Leading...
FDA clears AI-driven detection tool for pulmonary embolism
Medical imaging AI company Avicenna.AI has announced 510(k) clearance from the US Food and Drug Administration (FDA) for its CINA-iPE artificial intelligence (AI)-powered tool that...
CLOUT registry: Interim two-year rates of PTS ‘significantly lower’ than those...
Rates of post-thrombotic syndrome (PTS) among deep vein thrombosis (DVT) patients treated with the ClotTriever thrombectomy system (Inari Medical) who are logged in the...
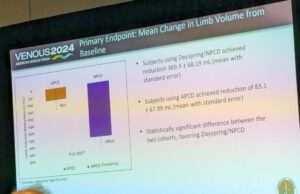
Portable non-pneumatic compression device shows superiority over advanced pneumatic device in...
A new study presented during the 2024 American Venous Forum (AVF) in Tampa, Florida (March 3–6), that compared a novel non-pneumatic compression device with...
SCAI, SIR, SVS jointly publish proceedings from multispecialty peripheral IVUS roundtable
Proceedings from an expert consensus roundtable that discussed the benefits of intravascular ultrasound (IVUS) in lower extremity revascularization procedures were released today in the...
Enrollment complete in APEX-AV study of mechanical aspiration system for acute...
Patient enrollment is now complete in the APEX-AV trial evaluating the safety and efficacy of the AlphaVac F1885 (AngioDynamics) multipurpose mechanical aspiration system for...
Computer-assisted vacuum thrombectomy system for patients with pulmonary embolism demonstrates ‘rapid,...
Patients undergoing computer-assisted vacuum thrombectomy with the Indigo Aspiration System (Penumbra) for pulmonary embolism (PE) showed 2.7% rates of both major adverse events (MAEs)...
Six-month outcomes from phase-one trial of anti-inflammatory drug to assess benefit...
The open-label phase of the DEXTERITY-AFP trial investigating the Bullfrog microinfusion device—which involves the perivenous injection of the anti-inflammatory drug dexamethasone to improve patency...
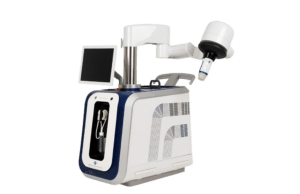
FDA approves IDE for pivotal study of high-intensity focused ultrasound platform
The Food and Drug Administration (FDA) has approved an investigational device exemption (IDE) application for the VEINRESET multicenter pivotal study that will evaluate Sonovein...
Venous stenting: “We need to evaluate use, appropriateness and clinical indication”
An analysis of venous stent usage trends in the USA from 2014 to 2021 showed a “significant increase” in stents per day placed over...
AVF 2023: Results from first US trial of Sonovein delivered
The 2023 annual meeting of the American Venous Forum (AVF; 22–25 February, San Antonio, USA) heard the final results from the first US trial...
AVF 2023: FLASH registry suggests “favourable safety and effectiveness profile” in...
Among 61 high-risk pulmonary embolism (PE) patients followed through to the 30-day visit in the US cohort of the FLASH registry, no mortalities were...
AVF 2023: One-year analysis of CLOUT registry reveals “significant and sustained”...
Interim one-year outcomes from the multicentre, prospective, single-arm CLOUT registry investigating use of the ClotTriever thrombectomy system (Inari Medical) in all-comer patients with deep...
AVF 2023: Investigators report update on three-year first-in-human results for bioprosthetic...
Researchers in Colombia behind the first-in-human study of a novel bioprosthetic venous valve designed to treat chronic venous insufficiency (CVI) reported three-year results among...
New study indicates cost-effectiveness of Denali IVC filter over Option device
Tilting or hooking occurred significantly less often in the process of retrieving the Denali inferior vena cava (IVC) filter (BD) than with the Option...
Overtreatment in venous disease: Financial incentives are “the elephant in the...
To combat the “overtreatment problem” in the appropriate care of venous disease, “a concerted, complex, multimodal effort” is required from specialists across disparate parts...
AVF 2022: Emerging autogenous venous valve formation system sees “continual improvement”
An emerging endovenous valve formation system designed to treat patients with chronic venous insufficiency (CVI) with evidence of deep venous reflux has demonstrated continual...
AVF 2022: Six-month CLOUT data indicate ClotTriever can effectively remove full...
Six-month outcomes from the ongoing CLOUT registry demonstrate the “safety and efficacy” of the ClotTriever thrombectomy system (Inari Medical) in a real-world deep vein...
AVF 2022: VenoValve improvement “maintained” for 2.5 years without adverse events
Envveno Medical announced positive 30-month data from the first-in-human trial of the VenoValve bioprosthetic potential venous valve replacement during the 2022 American Venous Forum...
Registry data show greater clot chronicity on postprocedure inspection than initially...
Nearly half of patients deemed preprocedurally to be suffering an acute case of deep vein thrombosis (DVT) turned out to have a “much more”...
Elder statesman of venous disease delivers golfing metaphor for fortitude, wellness
As a podium talk at the annual meeting of the American Venous Forum (VENOUS 2020; 3–6 March, Amelia Island, USA), it represented a bit...