Éva Malpass
Boston Scientific announces agreement to acquire Penumbra
Boston Scientific and Penumbra today announced the companies have entered into a definitive agreement under which Boston Scientific will acquire Penumbra in a cash...
Inquis Medical closes series C financing round
Inquis Medical has announced the closing of a US$75 million series C financing round.
The company have stated in a recent press release that the...
Gore announces US FDA approval for Fortegra venous stent
Gore has announced the US Food and Drug Administration (FDA) approval of the Gore Viabahn Fortegra venous stent—previously known as the Gore Viafort vascular...
Venous News’ top stories of 2025
Which stories were most popular among the venous community in 2025? Read our round-up of the trending articles which appeared across Venous News this year.
1....
New Medicare analysis shows increased thrombectomy use in venous disease
A new review of Medicare data shows a marked increase for both arterial and venous thrombectomy claims for venous thromboembolic disease filed between 2017–2022.
Researchers...
Study finds PERT-advised mechanical thrombectomy reduces mortality in PE
Results from a retrospective observational cohort study demonstrate reduced 30-day all-cause mortality, length of hospital stay and supplemental oxygen use at discharge in patients...
Study reveals elevated VTE risk in patients with rheumatoid arthritis
Findings from a large population-based cohort study have shown that individuals with rheumatoid arthritis (RA) are at an elevated risk of venous thromboembolism (VTE)...
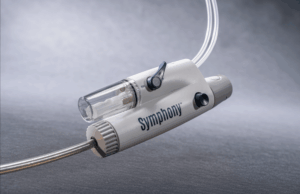
Imperative Care receives US FDA 510(k) clearance for Symphony thrombectomy system
Imperative Care today announced US Food and Drug Administration (FDA) 510(k) clearance of its Symphony thrombectomy system to treat pulmonary embolism (PE).
This clearance expands the...
Study finds high-protein diet increases risk of cancer-associated VTE
A new study based on an experimental model has shown that a high-protein or tryptophan (Trp)-rich diet increases the risk of cancer-associated venous thromboembolism...
Pathways and multidisciplinary teams “critical” in shaping future venous care
At this year’s Charing Cross (CX) Symposium (23–25 April, London, UK), Manj Gohel (Cambridge University Hospitals, Cambridge, UK) spoke with Stephen Black (Guy’s and...
The evolving role of vascular surgery in the treatment of severe...
Raghu Motaganahalli (Indiana University School of Medicine, Indianapollis, USA) discusses the expanding space of surgical treatment of severe chronic venous insufficiency (CVI), novel technologies...
Inari Medical, now part of Stryker, launches InThrill thrombectomy system
Inari Medical—now part of Stryker—has today announced the launch of its next-generation InThrill thrombectomy system.
The company has stated in a recent press release that...
Embolization receives US FDA 510(k) clearance for its NED embolisation device
Embolization recently announced it has recevied 510(k) clearance from the US Food and Drug Administration (FDA) for its Nitinol Enhanced Device (NED)—a vascular embolisation device intended for...
Endovascular Engineering’s ENGULF pivotal trial completes patient enrolment
Endovascular Engineering (E2) has completed patient enrolment in the pivotal cohort of its ENGULF trial, involving the Hēlo pulmonary embolism (PE) thrombectomy system.
The investigational...
Artificial intelligence tool predicts DVT risk following endovenous thermal ablation
A recent research article has detailed an artificial intelligence (AI) model that enables physicians to predict which patients with superficial venous insufficiency have a...
Three-year VenoValve first-in-human trial data published
Envveno Medical has today announced that its manuscript titled, ‘Three-year outcomes of surgical implantation of a novel bioprosthetic valve for the treatment of deep...
Imperative Care announces completion of enrolment in the SYMPHONY-PE study
Imperative Care today announced the completion of patient enrolment in its SYMPHONY-PE study, a pivotal investigational device exemption (IDE) trial evaluating the safety and...
JETi registry reports effective thrombus reduction in lower extremity DVT
Recently presented at the Society of Interventional Radiology (SIR) annual scientific meeting (29 March–2 April, Nashville, USA), data from the Jet Enhanced Thrombectomy intervention...
Kathleen Ozsvath
Kathleen Ozsvath (New York, USA), past president of the Eastern Vascular Society (EVS), is an esteemed vascular surgeon with expertise in the treatment of...
Akura Medical enrols first patient in QUADRA-PE study
Akura Medical has announced today the first patient enrolment in the QUADRA-PE study evaluating the Katana thrombectomy system in patients with acute pulmonary embolism...
Large survey in Japan finds cyanoacrylate closure for varicose veins “safe”...
A large nationwide survey conducted by the Japanese Regulatory Committee for Endovascular Treatment of Varicose Veins has demonstrated that cyanoacrylate closure (CAC) for varicose...
US FDA announces plans to address medical device shortage risks
The US Food and Drug Administration (FDA) has issued a statement outlining measures to enhance protections against medical device shortages.
According to Michelle Tarver,...
Piccolo Medical announces distribution agreement with Spectrum Vascular
Piccolo Medical has announced an exclusive distribution agreement with Spectrum Vascular. Spectrum Vascular offers a portfolio of venous catheters that leverage the Spectrum antimicrobial...
Argon Medical enrols first patient in CLEAN-PE study
Argon Medical announces the first patient enrolment in the CLEAN-PE study. The prospective, multicentre CLEAN-PE study aims to evaluate the safety and efficacy of...
US FDA issues first draft guidance for AI-based medical device development
The US Food and Drug Administration (FDA) has issued draft guidance that includes recommendations to support development and marketing of safe and effective artificial...
Research supports combined treatment approach for erectile dysfunction due to mixed...
Data from a new study have found that venous leak embolization yields additional clinical improvement and treatment potential in patients with vasculogenic erectile dysfunction...
Inari Medical’s ClotTriever thrombectomy system gains reimbursement approval in Japan
Inari Medical has announced that it received national reimbursement approval from the Japanese Ministry of Health, Labor and Welfare (MHLW) for its ClotTriever thrombectomy...
Inquis Medical announces completion of US$40 million series B financing
Inquis Medical has announced the successful closure of its US$40 million series B financing round. This round was led by Marshall Wace, a globally...
Zilver Vena stent shows three-year patency across patient groups
Cook Medical’s Zilver Vena venous self-expanding stent has shown high rates of patency sustained for three years, according to recently published data in the Journal of Vascular...
Sonablate announces first patient enrolled in clinical trial for ablation of...
Sonablate has announced the first patient enrolled in the HIFIVE clinical trial in the USA under the guidance of principal investigator, Naiem Nassiri (The...
Mechanical thrombectomy shifts the needle toward interventional management of PE and...
This advertorial is sponsored by Inari Medical.
Evaluating the current standard of care for the management of acute pulmonary embolism (PE) and deep vein thrombosis...
Research suggests potential link between strenuous physical activity and increased VTE...
Published in the Journal of Thrombosis and Thrombolysis, new research with a follow-up of 27 years has found an association between high levels of...
Surmodics receives US FDA 510(k) clearance for Pounce XL thrombectomy system
Surmodics has announced that it has received US Food and Drug Administration (FDA) 510(k) clearance for its Pounce XL thrombectomy system.
The Pounce XL thrombectomy...
B Braun receives US FDA clearance for deep access intravenous catheter
B Braun recently announced that the US Food and Drug Administration (FDA) has granted 510(k) clearance for the Introcan Safety 2 deep access intravenous...
Argon Medical announces launch of peripheral venous thrombectomy system
Argon Medical has today announced the launch of the Cleaner Vac thrombectomy system for the removal of blood clot from the peripheral venous vasculature.
The...
Deep venous stenting determined “safe” with low rates of major complications
A recent analysis of deep venous stent placement has determined that the procedure is safe with low rates of major complications. Published in the...
Study reveals improved COVID-19-associated VTE outcomes in post-vaccine period
A recent study published in the Journal of Thrombosis and Thrombolysis has revealed a significant decrease in the incidence of mortality and major bleeding in...
Jupiter Endovascular announces US FDA approval for SPIRARE II pivotal trial
Jupiter Endovascular has announced that the US Food and Drug Administration (FDA) has approved its Investigational Device Exemption (IDE) application for the SPIRARE II US pivotal study. The pivotal trial will...
Obvius Robotics receives US FDA Breakthrough Device designation for CERTA central...
Obvius Robotics has announced that the US Food and Drug Administration (FDA) has granted Breakthrough Device designation for its CERTA access system for central...
Prosthetic valve implantation shows “vast” improvements in deep chronic venous insufficiency
“The surgical challenges of repairing deep venous reflux have been present for more than a generation. It is worth reflecting on the fact that...
New randomised data show cyanoacrylate glue performs strongly on patient satisfaction
Patients undergoing cyanoacrylate glue closure for superficial venous disease reported higher periprocedural satisfaction than those who were treated via surgical stripping, data from a...
One-year results for synchronous anterior accessory saphenous vein ablation hold up
Twelve-month results from the SYNCHRONOUS trial, which is evaluating the role of prophylactic anterior accessory saphenous vein (AASV) ablation for the prevention of recurrent...
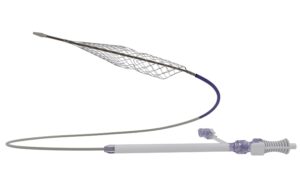
Penumbra announces FDA clearance of Lightning Flash 2 for the treatment...
Penumbra has announced the US Food and Drug Administration (FDA) clearance and launch of Lightning Flash 2, the next generation computer assisted vacuum thrombectomy (CAVT)...
Review urges development of protocols to reduce hypersensitivity reactions after cyanoacrylate...
Despite being considered “generally safe”, cyanoacrylate can cause local reactions in up to 25% of patients, disproportionately affecting women, Asian race and thin patients...
Biolitec announces two new devices in the ELVeS radial portfolio
Biolitec recently announced that it has extended its ELVeS Radial laser system for the minimally invasive laser treatment of insufficient veins by two further...
FLAME, FLASH and ongoing PEERLESS trials collect “compelling” data for FlowTriever...
Speaking on the FLAME, FLASH and PEERLESS trials that each collected data on the FlowTriever (Inari Medical) device for the treatment of pulmonary embolism...
JETi registry provides “remarkable results” for peripheral thrombectomy system
Presenting “remarkable results” from the JETi registry—a prospective, multicentre, observational study which collected real-world data on the safety, performance and clinical benefits of the...
Study finds anatomic positioning and physical activity type can cause device...
A recent multicentre, prospective study has found that stent deformations are greater in the common iliac vein with higher levels of hip flexion, as...
Analysis of varicose vein registry finds women benefit from endovenous ablation...
An evaluation of the Vascular Quality Initiative’s (VQI) Varicose Vein Registry (VVR) carried out by the Midwestern Vascular Surgical Society (MVSS) has found women...
Meta-analysis of PTS finds one in five risk of long-term symptoms...
A meta-analysis is first to report the pooled risk of post-thrombotic syndrome (PTS) after isolated distal deep vein thrombosis (DVT). Researchers revealed a one...
Boston Scientific announces investment to acquire majority stake of Acotec Scientific
Boston Scientific has announced that it will make a partial offer to acquire a majority stake, up to a maximum of 65%, of shares...
Real-world analysis confirms benefit of Xarelto in treating CAT as apixaban
Head-to-head observational analysis showed Xarelto as effective in treating cancer-associated thromboembolism (CAT) as apixaban.
The Janssen Pharmaceutical Companies of Johnson & Johnson have announced observational...