willdate
Haemonetics acquires vessel closure specialist Vivasure Medical
Haemonetics Corporation today announced the acquisition of Vivasure Medical, a Galway, Ireland-based company developing next-generation technology for percutaneous vessel closure.
Vivasure's PerQseal Elite system uses...
Akura Medical closes series C financing
Akura Medical has secured a US$53 million first close in series C financing, with the funds to be used to support development activities for...
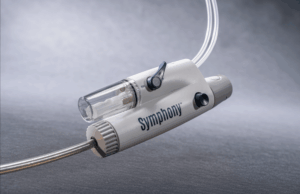
SYMPHONY-PE trial demonstrates positive efficacy, efficiency, and safety results
Imperative Care has announced efficacy and safety results from the pivotal SYMPHONY-PE trial evaluating the company’s Symphony thrombectomy system in the treatment of acute...
New medical device reporting requirements come into effect in the UK
New regulations have come into effect in the UK which place a greater emphasis on medical device manufacturers to monitor the safety and performance...
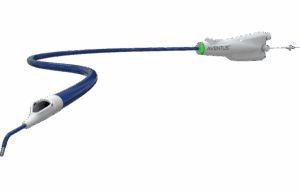
Aventus thrombectomy system gains FDA clearance for PE treatment
Inquis Medical has announced that its Aventus thrombectomy system has received 510(k) clearance from the US Food and Drug Administration (FDA) for an expanded...
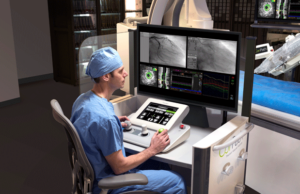
Stereotaxis seeks to broaden use of its robotic magnetic navigation system...
Stereotaxis has announced a US Food and Drug Administration (FDA) regulatory submission for the first robotically navigated catheter designed to expand usage of robotic...
US FDA approves IDE application for Katana thrombectomy system
The US Food and Drug Administration (FDA) has approved Akura Medical’s investigational device exemption (IDE) application to initiate the QUADRA-PE study evaluating the Katana...
US FDA approves ENGULF US pivotal trial of Hēlo thrombectomy system
Endovascular Engineering (E2) has announced US Food and Drug Administration (FDA) investigational device exemption (IDE) approval for its ENGULF US pivotal trial.
The study will...
UK MHRA adds capacity for medical device certification
The UK’s Medicines and Healthcare products Regulatory Agency (MHRA) has designated three new approved bodies to increase the country’s capacity to certify medical devices.
TÜV...
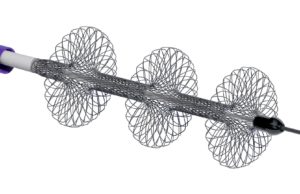
ClotTriever “makes fast, safe and efficient thrombectomy possible”
This advertorial is sponsored by Inari Medical
“We need to eliminate symptoms as fast as possible—it is not OK just to make things a little...
US FDA seeks to “modernise” clinical trials with new draft guidance
The US Food and Drug Administration (FDA) has released draft guidance with updated recommendations for good clinical practices (GCPs) aimed at modernising the design...
Amazon executive joins Medtronic to spearhead development in robotics and implantables
Medtronic has announced that Ken Washington has been appointed chief technology and innovation officer.
Washington joins Medtronic from Amazon where he served as vice president...
SCAI 2023: Mechanical thrombectomy improves symptoms and quality of life in...
Six-month outcomes from the FLASH registry have shown that patients with pulmonary embolism who were treated with mechanical thrombectomy showed significant improvement in symptoms,...
EU ministers approve changes to MDR transition timetable
The European Union’s Council of Ministers has today adopted a resolution to extend the deadline for the certification of medical devices under the Medical...
ACC 2023: Large bore mechanical thrombectomy reduces adverse outcomes in high-risk...
Large bore mechanical thrombectomy with the FlowTriever system (Inari Medical) in patients with high-risk pulmonary embolism (PE) was associated with a significantly lower occurrence...
Corindus rebrands to Siemens Healthineers Endovascular Robotics
Corindus has been rebranded to Siemens Healthineers Endovascular Robotics and will sit as a dedicated business within the Advanced Therapies area of Siemens Healthineers,...
EU Commission to propose delay to MDR implementation
Europe’s health commissioner, Stella Kyriakides, has announced that proposals to extend the transition period for the implementation of the European Union’s (EU) Medical Device...
Medtronic expands reach of AI-powered surgical video management and analytics platform
Medtronic has announced that it has entered into a contract with Vizient to add Touch Surgery Enterprise, an AI-powered surgical video management and analytics...
First patient enrolled in PEERLESS study of FlowTriever system
Inari Medical has announced that the first patient has been enrolled in PEERLESS—a prospective, randomised controlled trial (RCT) comparing the outcomes of patients with...
Philips expands haemodynamic assessment in its Lumify handheld ultrasound system
Royal Philips has announced an update to its handheld ultrasound platform—Lumify—adding Pulse Wave Doppler technology to expand the haemodynamic assessment and measurement capabilities of...
FDA clears CavaClear IVC filter removal laser sheath
Royal Philips has announced US Food and Drug Administration (FDA) de novo clearance for the Philips inferior vena cava (IVC) filter removal laser sheath—CavaClear—to...
Medtronic announces 2045 net zero emissions ambition to combat climate change
Medtronic has announced its ambition to achieve net zero carbon emissions by fiscal year 2045 across its operations and value chain to accelerate efforts...
MDR comes into effect across EU
The European Union (EU) Medical Devices Regulation (MDR) takes effect from today (26 May 2021).
The Regulation revises quality and safety standards and the...
Joseph Caprini receives first CX Venous Lifetime Achievement Award, as speakers...
As evolving treatment modalities offer the potential for improved outcomes in patients with deep venous disease, the importance of patient selection and the need...
CX 2021 global panel advocates multidisciplinary approach to “badly treated” severely...
An international panel of venous and lymphatic experts has called for a multidisciplinary approach to identify the causes of severely swollen lower limbs and...
FDA clears Transit Scientific’s XO Score PTA scoring sheath platform
Transit Scientific has received US Food and Drug Administration (FDA) clearance for the XO Score percutaneous transluminal angioplasty (PTA) scoring sheath platform for use...