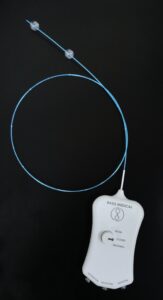
Basis Medical has announced the successful completion of its first-in-human clinical procedures using the Seclusion catheter to treat chronic venous insufficiency (CVI) caused by superficial vein reflux. The cases were performed by Marcos Fletcher (Ciudad de Salud Hospital, Panama City, Panama).
A press release notes that the Seclusion catheter is a next-generation, dual-balloon device engineered to deliver targeted therapy to malfunctioning superficial veins. It offers a non-thermal, non-tumescent approach to vein ablation, designed specifically to treat superficial venous reflux and CVI in the legs.
The Seclusion catheter received 510(k) clearance from the US Food and Drug Administration (FDA) in March 2025.
Fletcher commented: “The Seclusion catheter represents a significant advancement in the treatment of superficial vein disease. It delivered excellent control, precision, and patient comfort. We successfully treated target segments both above and below the knee, and the early results are highly encouraging. This technology has the potential to set a new standard in vein care.”
The cases were performed on patients suffering from symptomatic CVI and visible varicosities due to reflux in the great saphenous vein. Basis Medical shares that all patients tolerated the procedure well, and that no complications were reported during or immediately following treatment.
Tomas Levinton, chief executive officer of Basis Medical, added: “This is a pivotal moment for Basis Medical. We are extremely pleased with the catheter’s performance in these initial cases. These successful first-in-human results validate our approach and lay the groundwork for broader clinical adoption in both the US and international markets.”
To support its upcoming US commercial launch and advance clinical trials, Basis Medical is currently raising a US$6 million Series A financing round.