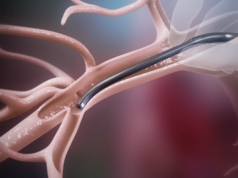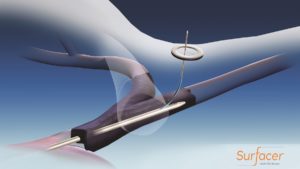
Bluegrass Vascular Technologies (Bluegrass Vascular) has announced the publication of positive results associated with the clinical use of the Surfacer Inside-Out access catheter system in the peer-reviewed Journal of Vascular Access (JVA).
Results of an independent, single-centre study demonstrated successful use of the Surfacer system in placement of haemodialysis catheters for patients with central venous obstruction involving one or more central veins. The mean time for placement and associated fluoroscopy was 13.3 and 3.7 minutes, respectively.
“The Surfacer system continues to be a safe and effective solution for accessing obstructed central veins,” states Dirk Hentschel, co-author of the publication and physician proctor for the company’s FDA approved US Investigational Device Exemption (IDE) clinical study. “This is of particular concern and importance for hemodialysis patients who are prone to life-threatening obstructions at vital access points.”
The newly published study in JVA adds to the growing body of evidence demonstrating the Surfacer system is a safe and reliable option for achieving right-sided central venous access and a viable means to preserving secondary central veins. Additional evidence resulting from the successful use of the Surfacer system haemodialysis patients awaiting the creation and/or maturation of an arteriovenous (AV) fistula was also recently reported in Hemodialysis International.
The company recently filed a de novo request for the Surfacer ystem with the US Food and Drug Administration (FDA) based on the positive clinical results of the company’s SAVE-US pivotal trial. The company anticipates that FDA will grant the request and the Surfacer system will be commercially available in the USA in 2020.