This advertorial is sponsored by Bentley.

Launched in July 2020, the BeYond Venous self-expanding stent system (Bentley InnoMed GmbH) now has early clinical data and expert experience to support its use in venous interventions. In what Michael Lichtenberg (Arnsberg Vascular Center, Arnsberg, Germany) describes as the “emerging” field of venous interventions, BeYond provides physicians with a strong stent option, offering in particular the best balance of the key attributes of high radial force, flexibility, and crush resistance.
In this interview with Vascular News, Lichtenberg outlines his early experience with BeYond, shares some newly released interim results with the device, and outlines what is next for this dedicated venous stent.
Could you give a brief overview of the field of venous interventions? Which patients are treated and why?
The venous field is certainly an emerging one. More and more patients with acute and chronic deep venous disease—including those with acute iliofemoral deep vein thrombosis (DVT) and chronic DVT, as well as those with May-Thurner syndrome—are being treated. Awareness is definitely rising, and not only on the patient side, but also on the physician side, which has meant that intervention numbers have increased over the last few years. I believe this trend will continue, as increasing numbers of patients are now referred for venous interventions.
What is the role of dedicated venous stents?
I think all specialists in the field align on the point that we need dedicated venous stents for treatment of acute and iliofemoral DVT because they have certain features, such as high radial force and flexibility, which uncovered, self-expandable arterial stents do not have. Without these attributes, stents may be crushed and may occlude very quickly, leading to disaster in terms of reocclusion, restenosis, and probably another DVT.

What is your early experience with the BeYond Venous stent?
At the Arnsberg Vascular Center, we have been using this stent for around one and a half years now. I would say I have gotten very used to this stent over the last few months, and I think it is a very good stent in terms of radial force, crush resistance, and flexibility. Based on our data, I can confidently say that this stent works well in the iliacs, and gives a very good answer to the stress which is present in the iliacs. From a clinical perspective, I believe the BeYond device is a very good stent option.
Is there one main advantage that you think makes the BeYond stent stand out in the venous stent market?
One feature that really stands out of that you can use the stent proximally, so it can go through the common iliac vein to the common femoral vein, thanks to the device’s radial force and flexible characteristics. To date, we have not experienced any issues with restenosis in either the common femoral vein or the distal external iliac vein, which is really positive. We have experienced this issue with other stents, and so it would seem that this stent is a very good compromise in terms of radial force on the one hand and flexibility on the other.
You recently conducted a retrospective analysis of 59 patients. What are the key takeaway messages from this analysis?
This cohort includes patients who were treated in our clinic over the last 18 months. We usually see patients on a regular basis after stent implantation, and so we have very good follow-up data. At 12 months, we have follow-up data for half of the patients. We only had four significant incidences of restenosis, which is a good chronic patency rate if you compare the data with those from other studies. Therefore, I must say that from a clinical standpoint this cohort analysis really proves the efficacy and safety of the stent. We included patients with non-thrombotic iliac vein lesions (NIVLs) and post-thrombotic syndrome (PTS), so it is a mixed cohort, a real-world cohort, with all the challenges we face in patients with acute and chronic disease.
How do you anticipate the BeYond stent might perform in the long term?
This is of course a little bit speculative, as there are many factors that influence long-term behaviour and effects of a venous stent, but I am feeling very positive about the results so far. We have not seen any significant problems yet, but we will have to wait for the two-year follow-up report, which we will hopefully get very soon.
If a colleague were to ask you about the BeYond venous stent, how would you describe it in three phrases?
The really positive aspects, besides excellent crush resistance and flexibility, are accurate and safe deployment, effective treatment, and good clinical data.
What is next for the BeYond stent?
We are currently working on two-year and also three-year follow-up data. We should have the two-year data in the second half of the year maybe in November or December, and of course three-year data in 2023.
Images from the case of a 42-year-old female patient with post-thrombotic syndrome after iliofemoral DVT 10 years before on the left side.
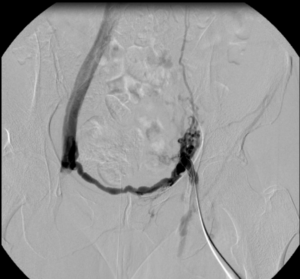
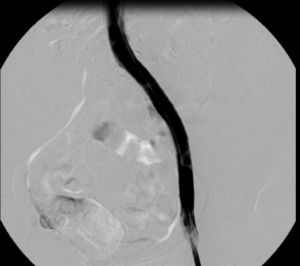
14x150mm BeYond Venous stent.
Disclaimer: The BeYond Venous stent is not FDA approved and is not for sale in the USA.