Vascular Breakthroughs and Vascular Care Connecticut have announced the first New England enrolment into the novel DEXTERITY clinical trials of local anti-inflammatory therapy to improve outcomes of deep vein thrombosis (DVT) therapy.
Paul Gagne and the research team at Vascular Breakthroughs and Vascular Care Connecticut have reached a key clinical development milestone in their participation in the DEXTERITY clinical trials with enrolment of their first patient, a press release reports. These trials, sponsored by Mercator MedSystems, are testing the delivery of an anti-inflammatory steroid to the tissue surrounding veins affected by DVT.
The patient treated by Gagne was enrolled into the DEXTERITY-AFP trial, designed to examine the use of the steroidal treatment in patients who have had symptoms for less than 14 days. A similar trial, the DEXTERITY-SCI trial, is being conducted to test the therapy in patients who have had symptoms longer, for 14–60 days.
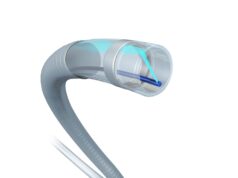
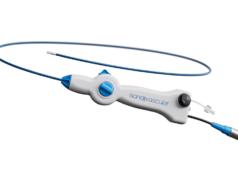
The procedure uses a cardiovascular catheter called the Bullfrog microinfusion device to precisely deliver the drug to the target tissue after clot has been removed by the physician via another small catheter placed in the vein.
“Overall, the procedure is very straightforward and easy to perform. The drug is visible under X-ray as we inject it, so we can immediately see the result of where we have treated,” said Gagne. “The inflammation caused by DVT can lead to chronic poor outcomes for these patients, so I look forward to seeing the outcomes of this trial as we continue to enrol.”