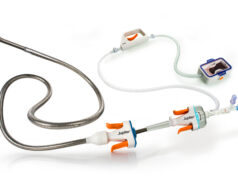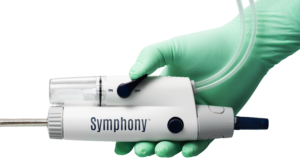
Imperative Care today announced US Food and Drug Administration (FDA) 510(k) clearance of the 82cm version of its Symphony 16Fr catheter, the company’s latest innovation designed to elevate care for patients with venous thrombosis.
“Thrombectomy procedures have emerged as one of the most powerful treatments in medicine, restoring vital blood flow in minutes. Historically, peripheral vascular thrombectomy has lacked technologies that effectively address challenging clot and preserve vessel health for the patient,” said Doug Boyd, senior vice president and general manager of Imperative Care’s Vascular business. “Imperative Care set out to transform vascular care through purpose-built precision thrombectomy technologies that combine large-bore power with a precisely controlled deep vacuum within the sterile field, along with a focus on minimising blood loss. The Symphony thrombectomy system has introduced precision thrombectomy to the market, and we are confident it will deliver faster, safer and more precise clot removal that will ultimately benefit patients impacted by devastating vascular diseases.”
Initial cases
The company also announced the successful completion of initial patient cases using the Symphony 16Fr 82cm catheter. Several of these were completed by Bennet George, interventional cardiologist at Vital Heart & Vein in Houston, USA.
“In my early experience, I’ve been able to consistently achieve 97% clot removal in, on average, two passes, with minimal blood loss (<120ml) in less than ten minutes. With a large-bore catheter and powerful deep vacuum, Symphony allows me to effectively treat highly challenging clot morphologies in minutes and in a blood-efficient way, ensuring the best possible outcomes for my patients,” said George. “The customised length of the 16Fr 82cm catheter gives me an additional capability to address both acute and organised clot in my venous procedures, and I look forward to its continued impact on the patients we treat.”
This latest innovation expands the Symphony thrombectomy system offering to include 16Fr 82cm, 16Fr 117cm and 24Fr 85cm catheters.
Imperative Care details that Symphony has an integrated controller that delivers nearly three times the clot removal force compared to legacy aspiration systems. Additionally, the system includes the ProHelix Mechanical Assist, a unique tool designed to facilitate clot ingestion, along with the Imperative Care Generator, which the company claims is the most powerful aspiration pump available on the market.
“At Imperative Care, we have always started by looking at critical unmet needs across the patient journey and working with a connected-care mindset to purposefully engineer solutions that put patients in the best position to live full and independent lives,” said Fred Khosravi, chairman and chief executive officer of Imperative Care. “With this latest offering and the enabling innovations in development at Imperative Care, we are poised to further advance the paradigm of care and provide physicians with the technologies that allow them to swiftly address these urgent ischaemic diseases and best care for their patients.”