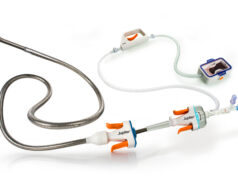
Inari Medical, a venture capital backed medical device company focused on the interventional treatment of venous thrombus, has announced the treatment of the first patient with its ClotTriever thrombectomy system.
The system received 510(k) marketing clearance from the US Food and Drug Administration on 16 February for the non-surgical removal of thrombus from the peripheral vasculature.
“We are pleased to be the first site to perform a procedure using the ClotTriever System. The ClotTriever removed clot in the iliac vein efficiently, and without the need for thrombolytic drugs,” said Mark Meissner, professor of Vascular Surgery at the University of Washington Medical Center in Seattle, USA. The ClotTriever is a promising new advancement in the treatment of thrombus in large veins,” added Sandeep Vaidya, associate professor of Interventional Radiology.
“The ClotTriever System is the second product offering in Inari’s product portfolio,” said Bill Hoffman, Inari’s chief executive officer. “The ClotTriever is currently available for treatment of thrombus in the peripheral veins at select centres, and we plan to add more centres as we gain clinical experience. We are pleased to provide a new tool for interventionalists to treat patients with large blood clots in big vessels, while avoiding the need for, and risk of, thrombolytic drugs,” added Hoffman.