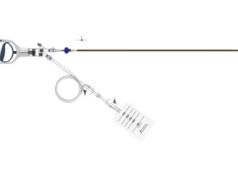
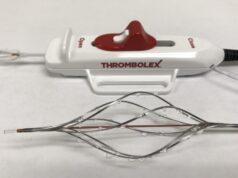
Intrinsic Imaging has been awarded a clinical trial to study an interventional medical device designed for the prevention of pulmonary emboli.
Throughout this trial, Intrinsic Imaging will provide imaging core lab services including, but not limited to, protocol and charter development, site qualification, site training and management, image transfer, blinding and processing as well as the specialised radiologic review of images.
Intrinsic Imaging says that it is the only imaging core lab that is ISO 13485 certified specifically to provide quality services for medical device clinical trials. The company’s team of full-time, board-certified, fellowship-trained interventional radiologists have experience assessing medical devices including, but not limited to, catheters, stents, angioplasty balloons, thrombectomy devices, venous access tools, novel devices and biologics.
As well as device assessment, Intrinsic Imaging guides in the development of the imaging charter and provide expertise in the selection of imaging modalities and acquisition protocols that are best oriented towards the placement, assessment and evaluation of medical devices.
Todd A Joron, president and chief operating officer, Intrinsic Imaging, said, “Acute pulmonary emboli are the most serious clinical presentation of venous thromboembolic disease and a major cause of hospitalisation, morbidity and mortality. Intrinsic Imaging brings sponsors unprecedented expertise in the management of medical device clinical trials.”
Intrinsic Imaging’s medical device certification was granted by the British Standards Institute.