 Global specialist healthcare company BTG has highlighted the commencement of the KNOCOUT PE study. The KNOCOUT PE study will measure how hospitals and patients are benefitting from a new standard of care in the treatment of pulmonary embolism utilising the company’s EKOS therapy with faster and safer protocols, following the encouraging results of the OPTALYSE PE study.
Global specialist healthcare company BTG has highlighted the commencement of the KNOCOUT PE study. The KNOCOUT PE study will measure how hospitals and patients are benefitting from a new standard of care in the treatment of pulmonary embolism utilising the company’s EKOS therapy with faster and safer protocols, following the encouraging results of the OPTALYSE PE study.
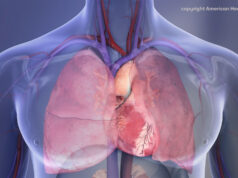
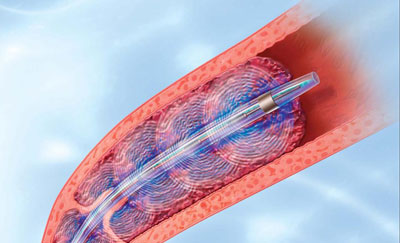
Presented in May 2016, the OPTALYSE PE study found that pulmonary embolism can be treated effectively with EKOS therapy over a much shorter period and at safer thrombolytic doses far below the previous standard. The EKOS system uses ultrasonic waves in combination with clot-dissolving thrombolytic drugs to effectively dissolve clots and restore healthy heart function and blood flow.
In clinical studies, EKOS therapy has been shown to speed time-to-clot dissolution, increase clot removal and enhance clinical improvement compared to either standard catheter-directed drug therapy or thrombectomy. EKOS therapy requires significantly shorter treatment times and less thrombolytic compared to standard catheter-directed drug therapy, lowering the risk of bleeding and other complications.
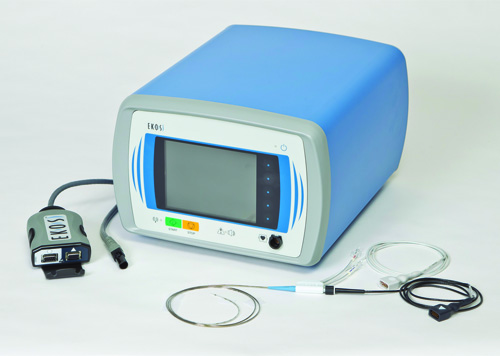
“We are excited to launch a large international registry of EKOS use including the duration of ultrasound and the dose of thrombolytic, in submassive PE. KNOCOUT PE permits a review of how interventionalists are implementing the OPTALYSE PE protocols and the results they are finding in real world practice,” said BTG vice president of Clinical Development Lynn Allen. “This importantly expands our clinical evidence to understand how clinical study findings such as these are adopted and what new best practices can be shared.”
At full enrolment, KNOCOUT PE is expected to include up to 100 centres internationally. Cases will include those from before and after the release of the OPTALYSE PE study.
“The results of the OPTALYSE PE trial suggest that a new standard for PE treatment might be adopted, the question is how are institutions adjusting to these new low dose, shorter duration treatments and what are they finding,” said KNOCOUT PE principal investigator Keith Sterling of Inova Alexandria Hospital, Alexandria, USA. “KNOCOUT PE accelerates the sharing and learning process and continues to build on the body of clinical evidence, including the ULTIMA and SEATTLE II studies.”
The OPTALYSE PE, ULTIMA and SEATTLE II studies were multi-centre trials examining ultrasound-facilitated, catheter-directed thrombolysis using a low dose of a standard clot dissolving medication called tissue plasminogen activator (tPA) to treat both acute massive and submassive pulmonary embolism. ULTIMA, a randomised controlled study comparing EKOS therapy to anticoagulation, looked at 59 patients across eight centres. SEATTLE II, a prospective single arm study, looked at 150 patients across 22 centres. OPTALYSE PE included 101 patients with acute proximal PE at 17 centres randomized to one of four treatment cohorts. The first cohort received 4mg of tPA per catheter over two hours. The second cohort received 4mg of tPA per catheter over four hours. The third cohort received 6mg of tPA per catheter over six hours. The fourth cohort received 12mg of tPA per catheter over six hours.
All cohorts saw a significant reduction in the main indicator of right heart strain from PE (measured as right ventricular to left ventricular diameter ratio (RV/LV)) by approximately 23 to 26 percent. The OPTALYSE PE results also showed a very low bleeding rate of three percent compared to 10 percent in the previous SEATTLE II study where patients were treated with 24mg for 12 or 24 hours.
The company has encouraged physicians seeking to participate in KNOCOUT PE or learn more, to contact their local BTG sales representative.