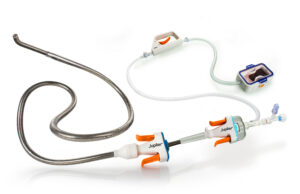
Penumbra unveils latest iteration of thrombectomy system for venous thromboembolism
Penumbra has launched the Lightning Flash 3.0 computer-assisted vacuum thrombectomy (CAVT) system, a development that includes "significant upgrades" to the venous thromboembolism (VTE) platform,...
Boston Scientific announces agreement to acquire Penumbra
Boston Scientific and Penumbra today announced the companies have entered into a definitive agreement under which Boston Scientific will acquire Penumbra in a cash...
Inquis Medical closes series C financing round
Inquis Medical has announced the closing of a US$75 million series C financing round.
The company have stated in a recent press release that the...
Haemonetics acquires vessel closure specialist Vivasure Medical
Haemonetics Corporation today announced the acquisition of Vivasure Medical, a Galway, Ireland-based company developing next-generation technology for percutaneous vessel closure.
Vivasure's PerQseal Elite system uses...