The US Centers for Medicare and Medicaid Services (CMS) has posted the 2018 Medicare Physician Fee Schedule (CMS-1678-F) and Hospital Outpatient Prospective Payment and Ambulatory Surgical Center Payment Systems (CMS-1678-FC). As part of the final rulings, healthcare providers will now be able to bill for VenaSeal utilising the new CPT codes 36482 and 36483. These new codes will take effect on 1 January, 2018.
Medtronic launched VenaSeal in the USA in 2015 following US Food and Drug Administration approval. Since then, VenaSeal has utilised a self-pay model, by which patients paid for the procedure out of pocket as reimbursement was limited. Together with clinicians, Medtronic has been working to demonstrate the clinical and economic benefits of the procedure and pursue payer coverage with major payers in the USA.
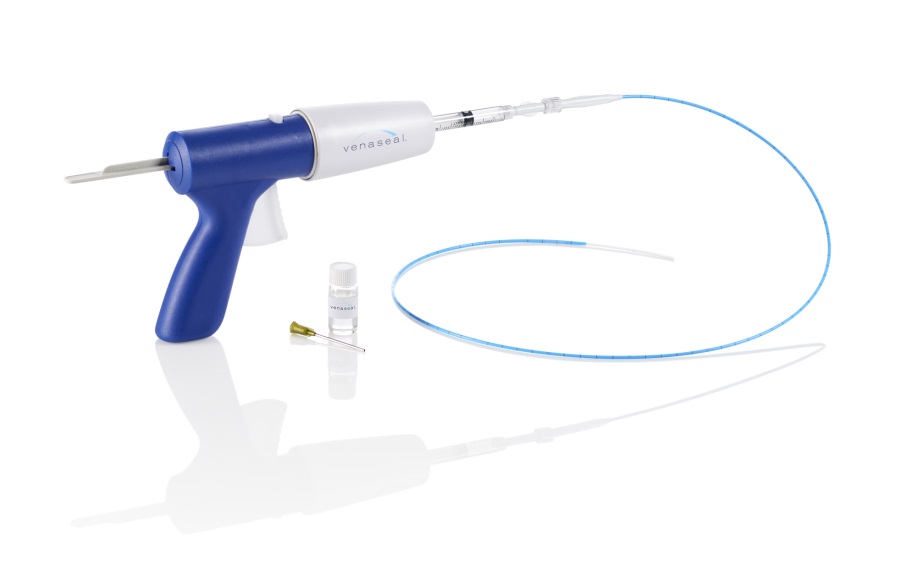
While there are other treatments on the market including radiofrequency and laser ablation, Medtronic states that the VenaSeal closure system is the only a non-tumescent, non-thermal, non-sclerosant procedure that uses a proprietary medical adhesive to close superficial veins of the lower extremities in patients with symptomatic venous reflux.
The VenaSeal closure system is supported by three-year data from the VeClose US pivotal clinical trial, which demonstrate long-term clinical and quality of life benefits. Furthermore, the VenaSeal closure system is designed to allow rapid recovery with minimal downtime and patients may not need to use compression stockings.
The “unique approach” of VenaSeal eliminates the risk of nerve injury that is sometimes associated with certain thermal-based procedures, according to a company press release. The procedure is administered without the use of tumescent anaesthesia, minimising the need for multiple needle sticks. Patients also report minimal-to-no bruising post procedure.









