Tag: Medtronic
Medtronic announces first commercial use of Liberant thrombectomy system
Medtronic has announced the first commercial use of its Liberant thrombectomy system, which is indicated for the removal of fresh, soft emboli or thrombi...
“We have two excellent treatments for superficial venous reflux,” The VEINS...
Sharing late-breaking secondary outcomes through 12 months from a randomised trial, Manj Gohel (Cambridge University Hospitals NHS Foundation Trust, Cambridge, UK) concluded at The...
ABRE subanalysis highlights obesity ‘paradox’ in post-venous stenting outcomes
Results from a subset analysis of the ABRE study have shown that patients in three out of the six World Health Organisation (WHO) body...
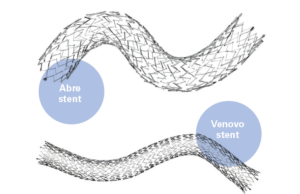
IVC 2024: Choosing the right stent for the right situation
Patrick Muck (Cinncinatti, Ohio) provided a state of the venous stent landscape during the 2024 International Vein Congress (IVC; April 18–20) in Miami.
The TriHealth...
Manj Gohel
“As I learned more about venous pathologies, it became abundantly clear that patients with venous disease were a largely neglected population in need of...
Nitinol stents placed in iliac veins “not associated with prolonged back...
In a retrospective review of over 600 patients who underwent iliac vein stent placement, the development of back pain was found to be unrelated...
Updated ClosureFast radiofrequency ablation catheter receives US FDA 510(k) clearance for...
Medtronic has announced that an updated ClosureFast radiofrequency ablation (RFA) catheter in a lower 6Fr profile is now available in the USA following 510(k)...
Amazon executive joins Medtronic to spearhead development in robotics and implantables
Medtronic has announced that Ken Washington has been appointed chief technology and innovation officer.
Washington joins Medtronic from Amazon where he served as vice president...
ABRE clinical study 36-month data show sustained effectiveness of Abre venous...
Medtronic has announced the 36-month final results from the ABRE clinical study. The purpose of the ABRE clinical study was to evaluate the safety...
Medtronic names Laura Mauri as new chief scientific, medical and regulatory...
Medtronic has announced that Laura Mauri has been appointed as the company’s chief scientific, medical and regulatory officer. This appointment adds to Mauri's prior responsibilities...
Abre venous stent “remains safe and effective” out to 24 months,...
The Abre venous stent (Medtronic) remains safe and effective for the treatment of iliofemoral venous obstructive disease out to 24 months. This is according...
Five-year, single-centre VenaSeal outcomes for symptomatic saphenous incompetence revealed
Morwan Bahi (Wellington Regional Hospital, Wellington, New Zealand) presented five-year results of a retrospective, observational, single-centre study detailing outcomes for all patients with symptomatic...
ABRE study meets primary safety and effectiveness endpoints
Medtronic has announced the first-ever results from the ABRE clinical study assessing the safety and effectiveness of the investigational Abre venous self-expanding stent system...
Omar Ishrak to step down as Medtronic CEO next year
Omar Ishrak, Medtronic’s chairman and CEO is to retire on 26 April 2020, following the end of the company’s current fiscal year. Also, the...
Non-thermal techniques for treatment of venous ulcers: Off-label use of cyanoacrylate...
At the American Venous Forum (AVF; 20–23 February, Tucson, USA), a session on venous leg ulcers turned to possible future treatment strategies as Kathleen...
IDE study to evaluate Abre Venous Self-Expanding Stent in patients with...
Medtronic has announced the initiation of its investigational device exemption (IDE) study for the Abre venous self-expanding stent system. The ABRE IDE Study will...
Medtronic receives favourable CMS ruling on VenaSeal
The US Centers for Medicare and Medicaid Services (CMS) has posted the 2018 Medicare Physician Fee Schedule (CMS-1678-F) and Hospital Outpatient Prospective Payment and...
Medtronic launches Concerto 3D detachable coil system in USA and Europe
Medtronic is expanding its embolisation product portfolio with the launch of the Concerto 3D detachable coil system. The system was launched at the Cardiovascular...
Adhesive closure “an effective add-on” to thermal ablation
Endovenous adhesive closure systems are a useful adjunct to thermal ablation treatment for varicose veins, but it is vital that the possible side-effects of...
VeClose trial indicates 24-month non-inferiority of VenaSeal versus radiofrequency ablation
Two-year outcomes of the VeClose pivotal trial have been reported, showing a 94.3% closure rate and “continued non-inferiority results to radiofrequency ablation” when using...
WAVES trial shows 100% vein closure at one month with short...
A post-market evaluation of the VenaSeal closure system (Medtronic) has found that, at one month, 100% of treated veins remained closed, quality of life...