Tag: SPIRARE II
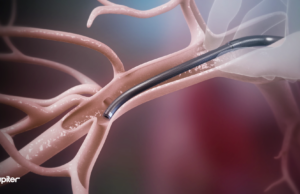
Jupiter Endovascular announce US FDA approval for SPIRARE II pivotal trial
Jupiter Endovascular has announced that the US Food and Drug Administration (FDA) has approved its Investigational Device Exemption (IDE) application for the SPIRARE II US pivotal study. The pivotal trial will...