Tag: SYMPHONY-PE
Imperative Care receives US FDA 150(k) clearance for Symphony thrombectomy system
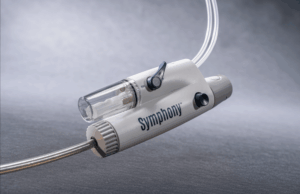
Imperative Care today announced US Food and Drug Administration (FDA) 510(k) clearance of its Symphony thrombectomy system to treat pulmonary embolism (PE).
This clearance expands the...
Imperative Care announces completion of enrolment in the SYMPHONY-PE study
Imperative Care today announced the completion of patient enrolment in its SYMPHONY-PE study, a pivotal investigational device exemption (IDE) trial evaluating the safety and...