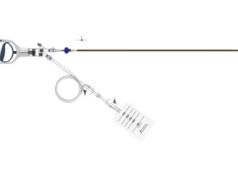
On 3 December, Volcano Corporation announced it has signed an agreement to acquire Crux Biomedical, developer of the Crux VCF System―an inferior vena cava (IVC) filter designed to prevent pulmonary embolisms.
The Crux VCF System, which is designed to facilitate bi-directional retrieval through either the femoral or jugular veins, received the CE mark in December 2011 and 510(k) FDA clearance in July 2012. In the RETRIEVE 2, 3 and 4 pivotal clinical trials, which enrolled 125 patients at high risk for pulmonary embolism across 22 global sites, the Crux VCF System demonstrated both deployment and retrieval success at 98% with 0% embolization, migration and fracture.
“The Crux VCF represents the first major design innovation for IVC filters in nearly four decades and incorporates important product attributes that provide improved patient outcomes,” said Thomas Fogarty, surgeon, founder of Crux Biomedical. “This transaction pairs two companies that have led innovation and I believe the future addition of IVUS guidance to the Crux technology will represent a seminal change in the practice of vascular medicine.”
Volcano announced that under the terms of the agreement it will pay US$36 million in cash at closing, which is expected on the second week of December 2012, subject to a working capital adjustment and customary closing conditions. Volcano also said that it will pay up to approximately US$3.1 million in Crux transaction expenses.
The merger agreement also provides for a potential post-closing cash payment of US$3 million upon FDA clearance of a 510(k) application submitted by Volcano on or before 30 June 2013 for a retrieval device currently being developed by Crux. In addition, Volcano may make additional cash payments for up to four years, based on sales of Crux products following their commercial launch.
Volcano announced it expects to initiate commercial sales of Crux products at the end of 2013 once full-scale manufacturing is implemented at its facility in California, USA. The company also plans to seek regulatory approval to market the Crux VCF System in combination with its IVUS (Intravascular Imaging) technology.
“We are delighted to be adding the novel Crux VCF System, as well as Crux’s other pipeline devices, to our innovative product portfolio. Our ability to provide this type of cutting-edge therapy furthers our leadership in the delivery of precision guided therapy and symbolises our continued evolution from an intravascular imaging company to one that provides a wide variety of diagnostic and therapeutic solutions,” said Scott Huennekens, president and CEO, Volcano.
“The Crux VCF System’s bi-directional retrievability makes it a highly valued solution in today’s environment of increased clinical focus on removing IVC filters when the risk of pulmonary embolism is reduced,” noted Neil Hattangadi, vice president and general manager of Volcano’s peripheral vascular unit. “This novel device, which is self-centering and naturally adjusts to the shape of the vessel wall, is a strong and synergistic addition that advances our presence in venous applications.”
“We are pleased that Volcano and Crux will be joining forces. Volcano has brought state-of-the art intravascular imaging technology to the global market and has clearly made a strong commitment to address the peripheral market,” said Randy Lindholm, chairman of Crux. “We believe Volcano is the perfect partner for us and is uniquely positioned to generate widespread adoption of the Crux VCF.”