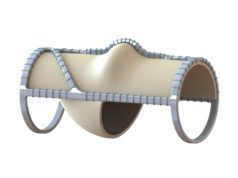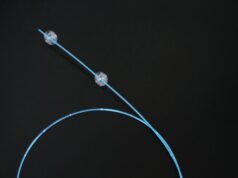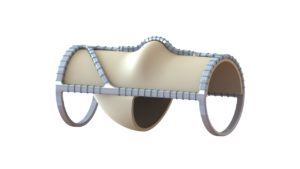
Hancock Jaffe Laboratories has announced that promising two-year post-VenoValve implantation data are being presented today at the Society for Vascular Surgery’s Vascular Annual Meeting (SVS VAM 2021; 18–21 August, San Diego, USA and online) by Jorge Ulloa (Fundacion Santa Fe-Universidad de los Andes, Bogota, Colombia), who was the principal investigator for the company’s first-in-human VenoValve trial.
Key highlights from Ulloa’s presentation indicate that:
- Reflux decreased from an average of 1.95 to an average of 0.72 (a 63% improvement)
- Disease manifestations (measured by revised venous clinical severity score [rVCSS]) decreased from an average of 13.38 to an average of 5.38 (a 60% improvement)
- Pain (measured by VAS scores) decreased from an average of 7.25 to an average of 0.5 (an improvement of 93%)
- There were no safety issues or venous ulcer recurrences
The data reported are for a group of eight patients who participated in the VenoValve first-in-human study and who agreed to participate in a one-year post-study follow-up. Three additional first-in-human patients elected to not participate in the one-year post-study follow-up, but reported no negative VenoValve-related events during the one-year follow-up period. The average post-VenoValve implantation time for this cohort of patients is two years, and the comparative results are based on pre-VenoValve levels compared to the patients’ most recent office visit.
Marc Glickman, Hancock Jaffe’s senior vice president and chief medical officer stated, “These data are exactly the results that we were looking for as we begin our VenoValve SAVVE [Surgical anti-reflux venous valve endoprosthesis] pivotal trial. Our patients are continuing to benefit from the VenoValve, with no safety issues and no ulcer recurrences. Chronic venous insufficiency [CVI] in the deep venous system has frustrated patients and physicians for decades and our primary investigators for the SAVVE study are as excited and enthusiastic as we are about the upcoming study.”
The SAVVE US pivotal trial for the VenoValve will include 75 patients at up to 20 sites. The primary endpoints for the SAVVE trial will be the same as for the first-in-human trial: the primary safety endpoint is the occurrence of a major adverse event (MAE) in less than 10% of patients at 30 days post-VenoValve implantation, and the primary effectiveness endpoint is improvement of reflux equal to or greater than 30% at six months following VenoValve surgery. MAEs are defined as the composite of all-cause mortality, deep wound infection, major bleeding, ipsilateral deep vein thrombosis (DVT), or pulmonary embolism. Improvement of VCSS and visual analogue scale (VAS) scores are also included in the SAVVE study as secondary endpoints.
As recently announced, the US Food and Drug Administration (FDA) has granted Breakthrough Device designation status to the VenoValve.