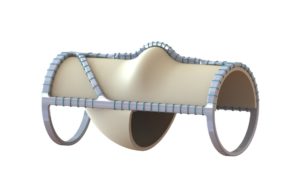
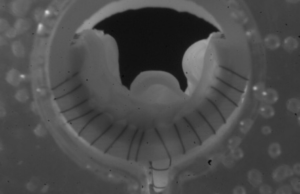
Tag: venovalve
Two-year data show continued clinical improvement with VenoValve
Implantation of the VenoValve (enVVeno Medical) continues to promote stabilisation of symptoms in patients with deep venous reflux at two-year follow-up. This is the...
Three-year VenoValve first-in-human trial data published
Envveno Medical has today announced that its manuscript titled, ‘Three-year outcomes of surgical implantation of a novel bioprosthetic valve for the treatment of deep...
VenoValve: Analysis finds similar improvement among both primary and thrombotic deep...
A new subanalysis of the SAVVE (Surgical antireflux venous valve endoprosthesis) trial found that there was no difference in the level of improvement in...
One-year data from VenoValve US pivotal trial highlighting impact on patients’...
One-year follow-up data on 75 subjects from the VenoValve (Envveno Medical) US pivotal trial were presented by principal investigator Cassius Iyad Ochoa Chaar (Yale...
One-year data from the VenoValve US pivotal trial emerge
Envveno Medical has announced that it will present one-year data on all patients from the VenoValve US pivotal trial today at the VEITHsymposium (19–23...
PMA application submitted for VenoValve device
enVVeno Medical today announced it has submitted an application with the U.S. Food and Drug Administration (FDA) seeking approval to market the VenoValve—a surgical...
Bioprosthetic venous valve imparts sustained clinical improvement at one year regardless...
A new paper that drills into the impact of a novel bioprosthetic venous valve replacement by CEAP (Clinical, Etiological, Anatomical and Pathophysiological) classification shows...
Prosthetic valve implantation shows “vast” improvements in deep chronic venous insufficiency
“The surgical challenges of repairing deep venous reflux have been present for more than a generation. It is worth reflecting on the fact that...
Deep venous valve technologies set to address “large unmet need worldwide”
The pursuit of a cure for deep venous valvular reflux—long considered to be the “holy grail” of deep venous disease—is underway, with new technologies...
AVF 2023: Investigators report update on three-year first-in-human results for bioprosthetic...
Researchers in Colombia behind the first-in-human study of a novel bioprosthetic venous valve designed to treat chronic venous insufficiency (CVI) reported three-year results among...
First-in-human patients continue to benefit from VenoValve at average of three...
Positive long-term, three-year observational data from a cohort of patients that participated in the previously concluded VenoValve (Envveno Medical) first-in-human clinical trial were recently...
AVF 2022: VenoValve improvement “maintained” for 2.5 years without adverse events
Envveno Medical announced positive 30-month data from the first-in-human trial of the VenoValve bioprosthetic potential venous valve replacement during the 2022 American Venous Forum...
Envveno Medical reports successful completion of first VenoValve surgery for US...
Envveno Medical, formerly Hancock Jaffe Laboratories, recently announced that the first VenoValve surgery in the company’s SAVVE US pivotal trial for the VenoValve has...
“The future is very bright for venous valve replacement”
The progress of two “promising” new devices in the venous valve replacement space were outlined during a special session at the Society for Vascular...
Hancock Jaffe presents positive two-year VenoValve data at SVS VAM 2021
Hancock Jaffe Laboratories has announced that promising two-year post-VenoValve implantation data are being presented today at the Society for Vascular Surgery’s Vascular Annual Meeting...
FDA grants Breakthrough Device designation status for Hancock Jaffe’s VenoValve
Hancock Jaffe Laboratories today announced that the US Food and Drug Administration (FDA) has granted Breakthrough Device designation status to the VenoValve, the company’s...
First US patent issues on Hancock Jaffe VenoValve
Hancock Jaffe Laboratories has revealed that the United States Patent and Trademark Office (USPTO) has issued the first patent covering the company's VenoValve. The...
Hancock Jaffe receives IDE approval to begin VenoValve US pivotal trial
Hancock Jaffe Laboratories recently announced that the US Food and Drug Administration (FDA) has approved the company's Investigational Device Exemption (IDE) application to begin...
Hancock Jaffe, maker of the VenoValve, completes US$41 million public offering
Hancock Jaffe Laboratories recently announced that it has completed a public offering of its securities, generating approximately US$41.4 million of gross proceeds, prior to...
Initial results from first-in-man trial of prosthetic VenoValve demonstrate promise
Initial results of an ongoing first-in-man study in Colombia, that saw the implantation of a prosthetic venous valve in 15 patients, have demonstrated an...
Endpoints for first-in-human VenoValve study announced by Hancock Jaffe
Medical device company Hancock Jaffe has announced the end-points for its upcoming VenoValve first-in-human study in Bogota, Colombia. Endpoints for the study will include...
Medical research committee gives approval for first-in-man VenoValve study
Hancock Jaffe Laboratories, a company specialising in bioprosthetic medical devices for treating cardiac and vascular diseases, has received approval for the first-in-man testing of...