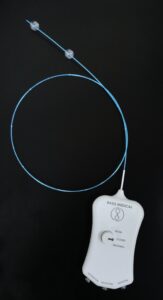
Basis Medical recently announced that its Seclusion catheter for superficial vein reflux has received US Food and Drug Administration (FDA) 510(k) clearance.
The company notes that the Seclusion catheter is an endovenous, chemical ablation device designed to provide a safer, more comfortable, and clinically effective solution for patients suffering from superficial vein reflux and chronic venous insufficiency (CVI). By utilising a dual-balloon system, the catheter temporarily isolates specific segments of diseased blood vessels, allowing for precise treatment with an FDA-approved sclerosant. This minimally invasive approach, the company claims, eliminates the need for thermal methods and tumescent anaesthesia, addressing the key limitations of current therapies.
“This FDA 510(k) clearance is a pivotal moment for Basis Medical,” said Tomas Levinton, chief executive officer, in the company’s press release. “The Seclusion catheter’s unique design enhances procedural efficiency while reducing risk and pain, benefiting both patients and physicians. We are excited to bring this technology to the market and continue our mission of delivering solutions that elevate patient care.”