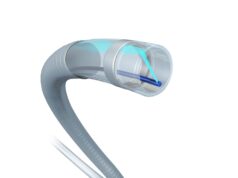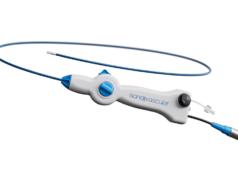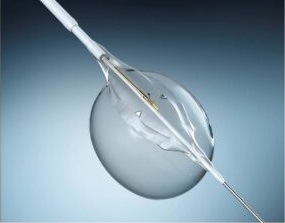
Mercator MedSystems has announced enrolment of the first patient in the DEXTERITY (Dexamethasone therapy examining reduction of inflammation after thrombus removal to yield benefit in DVT) Phase 2 clinical trial. Mercator’s proprietary Bullfrog micro-infusion device is a delivery solution designed to address local inflammation associated with the causes, the treatment, and the outcomes of deep vein thrombosis (DVT). In July 2021, Mercator received an investigational new drug (IND) approval for the two DVT studies from the US Food and Drug Administration (FDA).
According to Mercator, the Bullfrog device can potentially improve outcomes in peripheral vascular intervention for DVT by delivering an anti-inflammatory therapeutic agent directly around the affected vein, where it is needed most.
The DEXTERITY studies are intended to enrol a group of patients with acute femoropopliteal DVT (the DEXTERITY-AFP study) and another group with subacute or chronic iliofemoral DVT (the DEXTERITY-SCI study). Principal investigator David Dexter of Sentara Vascular Specialists treated and enrolled the first patient in the DEXTERITY-AFP study on 29 October 29 2021 at Sentara Norfolk General Hospital in Norfolk, USA.
“We have long recognised the need for successful and long-lasting outcomes for DVT patients. We are delighted to have initiated this important clinical trial for bringing local anti-inflammatory drug delivery to DVT interventions. The goal of this study is to show that local steroid treatment for DVT can address the inherent inflammation associated with DVT and improve the outcomes and durability after mechanical thrombus removal,” stated Dexter.
“Local drug therapy with thrombolytics has been used for years to remove thrombus from the legs, but the DEXTERITY studies represent the first locally targeted drug therapy designed to treat DVT inflammation. While it has long been an incredibly difficult problem in the treatment of DVT, it has been unaddressed until now,” said Trent Reutiman, chief executive officer of Mercator. “We are excited to be at the forefront of treatment for the devastating, chronic outcomes of DVT, showcasing the capabilities that come with efficient drug delivery using our portfolio of micro-infusion devices.”