Akura Medical has announced today the first patient enrolment in the QUADRA-PE study evaluating the Katana thrombectomy system in patients with acute pulmonary embolism (PE). The initial procedure was successfully performed by Samuel Horr, director of cardiovascular research at TriStar Centennial Medical Center in Nashville, USA.
Akura Medical has announced today the first patient enrolment in the QUADRA-PE study evaluating the Katana thrombectomy system in patients with acute pulmonary embolism (PE). The initial procedure was successfully performed by Samuel Horr, director of cardiovascular research at TriStar Centennial Medical Center in Nashville, USA.
“TriStar Centennial is thrilled to be a part of research trials that hold the potential to deliver better care to patients,” commented Horr. “With this trial, the hope is to provide physicians with continuous, real-time pulmonary artery pressure readings throughout the entire procedure to streamline the removal of the blood clot.”
“One of the biggest challenges in thrombectomy procedures for PE is determining when you’ve successfully removed the thrombus entirely, as incomplete removal can may lead to suboptimal outcomes,” said Sanjum Sethi (Columbia University Medical Center, New York, USA) co-principal investigator for the QUADRA-PE trial. “The Katana System’s display of real-time pressure data represents a significant advancement, providing physicians with clinically useful insights during the procedure.”
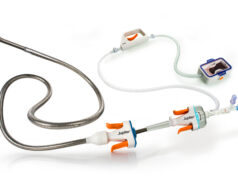
A recent press release shared by the company has stated that the Katana system includes:
- A bidirectional, low-profile sheath designed to facilitate smoother navigation in complex vasculature and enable contrast injection without requiring catheter exchanges.
- High velocity saline jets that are engineered to effectively break up clots independent of morphology and prevent catheter clogging for procedural efficiency.
- Sensors that provide real-time pulmonary artery pressure data to provide insights into procedure progress.
- The Sentinel console, which displays clot engagement and blood loss to inform the physician and to potentially minimise uncertainty.
“Pulmonary embolisms can be life-threatening and demand swift and effective intervention. Meaningful strides have been made in the thrombectomy landscape, but challenges remain in navigating complex vasculature and simplifying decision making,” said Ann Gage (TriStar Centennial Medical Center, Nashville, USA), co-principal investigator for the QUADRA-PE trial. “I am incredibly excited to be involved in this study as it represents a significant milestone in the forward trajectory of pulmonary embolism treatment.”
The QUADRA-PE study is a multicentre, international trial designed to enrol up to 118 patients with clinically significant acute PE, at up to 26 sites globally. The primary effectiveness endpoint is the reduction in right ventricular/left ventricular (RV/LV) ratio from baseline to 48 hours post-procedure as assessed by CT angiography. The primary safety endpoint is the composite rate of major adverse events (MAEs) within 48 hours post-procedure.