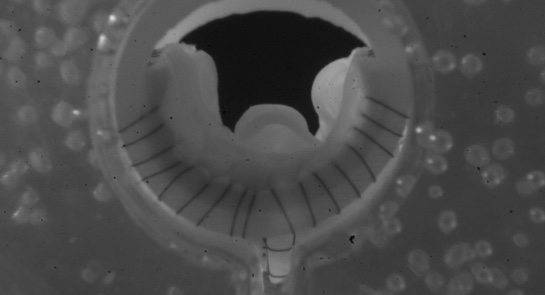
Hancock Jaffe Laboratories, a company specialising in bioprosthetic medical devices for treating cardiac and vascular diseases, has received approval for the first-in-man testing of its VenoValve bioprosthetic venous valve device from the Medical Research Committee at Fundación Santa Fe de Bogotá (FSFB), in Bogota, Colombia. The company previously received approval from the FSFB’s Ethics Committee.
Hancock Jaffe will now begin gathering the information necessary to submit an application for approval to Instituto Nacional de Vigilancia de Medicamentos y Alimentos (INVIMA), which is the Colombian equivalent of the US Food and Drug Administration. INVIMA approval is required to import investigational medical devices and conduct human clinical trials in Colombia.
Hancock Jaffe is developing the VenoValve to treat severe cases of chronic venous insufficiency. Approximately 4.5 million people in the USA suffer from severe chronic venous insufficiency, and the condition results from between 400,000 to 700,000 hospitalisations per year. There are currently no FDA approved treatments for deep venous chronic insufficiency.








