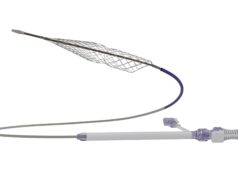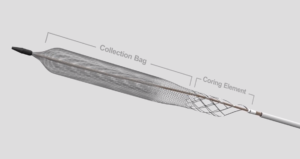
Inari Medical has announced strongly positive interim results of the first 64 chronic deep vein thrombosis (DVT) patients enrolled in the ClotTriever outcomes (CLOUT) registry. The subanalysis, which focused on patients with an estimated clot age over six weeks, was presented virtually at LINC 2021 (Leipzig Interventional Course; 25–29 January, virtual) by principal investigator, Steven Abramowitz (MedStar Health, Washington, DC, USA).
“Be it ongoing COVID fears, delays in diagnosis, or impediments to referral, many DVT patients have chronic disease by the time they finally meet a DVT interventionist. These physicians know that chronic clot is more wall-adherent and collagen-rich, rendering traditional lytic-based tools ineffective,” said Abramowitz.
“The CLOUT chronic subanalysis has shown us that ClotTriever can offer these chronic DVT patients hope, removing a median 90% of their clot in a single session without the use of thrombolytics.” No serious device-related adverse events were reported across the 14 subanalysis sites, median blood loss was a modest 50cc, and median thrombectomy procedure time was 34 minutes.
Also at LINC, Inari is hosting a live lunch symposium on Tuesday, January 26, at 12:30pm CET (6:30am ET), moderated by Gerard O’Sullivan (Galway Clinic, Galway, Ireland) including remarks on the latest Inari study experience by Abramowitz and Michael Jolly (OhioHealth, Columbus, USA).