Tag: inari medical
Why treat DVT interventionally with a “wall-to-wall” mechanical thrombectomy approach?
In a recent interview, Marie Josee van Rijn from Rotterdam, the Netherlands and Emma Wilton from Oxford, UK, provided insight into the transformative power...
Mechanical thrombectomy demonstrates superior outcomes to anticoagulation in DVT patients, analysis...
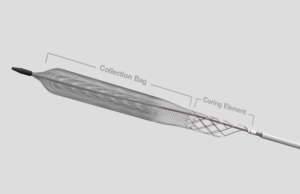
Mechanical thrombectomy using the ClotTriever device (Inari Medical) for iliofemoral deep vein thrombosis (DVT) was found to be “associated with significantly” lower Villalta scores...
DEFIANCE randomized controlled trial set to provide best assessment of mechanical...
NOTE: This video is ONLY available to watch in selected countries and geographies
David Dexter, (Sentara Vascular Specialists, Norfolk, USA, National Co-PI of the...
Venous News’ top stories of 2023
Which stories captured the attention of the venous community this year? Read our summary of the trending articles from across the Venous News network...
One-year CLOUT data demonstrate low rate of PTS following treatment with...
David Dexter (Sentara Vascular Specialists, Norfolk, USA) shared one-year data from the CLOUT registry this week at The VEINS 2023 (28–30 October, Las Vegas,...
REAL-PE demonstrates statistically significant lower major bleeding rates with Ekos system...
Data from the REAL-PE study were presented this week at TCT 2023 (23–26 October, San Francisco, USA) demonstrating that patients treated for pulmonary embolism...
Expanding the treatment window for the effective management of DVT
“The median thrombus age of DVT patients treated in our centre is 14 days – says Dr Andrew Wigham from Oxford University Hospitals. –...
Mechanical thrombectomy: ClotTriever offers “extended window” for DVT treatment
This advertorial, sponsored by Inari Medical, is only available in selected countries and geographies.
During a recent webinar hosted by Inari Medical, a multidisciplinary group...
ClotTriever “makes fast, safe and efficient thrombectomy possible”
This advertorial is sponsored by Inari Medical
“We need to eliminate symptoms as fast as possible—it is not OK just to make things a little...
Inari Medical announces commercial launch of RevCore and Triever16 Curve for...
Inari Medical today announced the launch of two new purpose-built products, the RevCore thrombectomy catheter, and the Triever16 Curve catheter.
According to a company press...
Why might CLOUT and DEFIANCE open the door to a new...
Steven Abramowitz (Washington DC, USA) explains the significance of the CLOUT registry—the only registry capturing data on mechanical thrombectomy in deep venous thrombosis (DVT)....
ACC 2023: Large bore mechanical thrombectomy reduces adverse outcomes in high-risk...
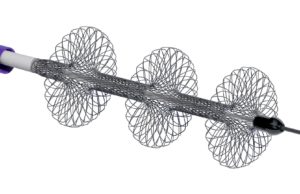
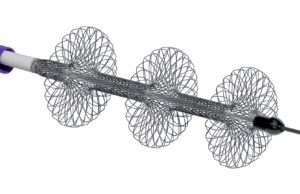
Large bore mechanical thrombectomy with the FlowTriever system (Inari Medical) in patients with high-risk pulmonary embolism (PE) was associated with a significantly lower occurrence...
AVF 2023: One-year analysis of CLOUT registry reveals “significant and sustained”...
Interim one-year outcomes from the multicentre, prospective, single-arm CLOUT registry investigating use of the ClotTriever thrombectomy system (Inari Medical) in all-comer patients with deep...
Inari Medical announces first patient enrolment in DEFIANCE trial of the...
Inari Medical has announced that the first patient has been enrolled in DEFIANCE, a prospective randomised controlled trial (RCT) comparing the clinical outcomes of...
Mechanical thrombectomy for DVT: Randomised data needed to boost growing evidence...
Two datasets presented during the late-breaking clinical trials session at The VEINS (Venous Endovascular Interventional Strategies) 2022 (30–31 October, Las Vegas, USA)—the latest results...
New mechanical thrombectomy systems unlock possibilities for the treatment of venous...
Gerd Grözinger (Tübingen, Germany) chats with Bernhard Gebauer (Berlin, Germany) at the Cardiovascular and Interventional Radiological Society of Europe (CIRSE) 2022 annual meeting (10–14...
FLASH results demonstrate “excellent safety profile” of the FlowTriever system in...
Results of the FLASH registry demonstrate the “excellent safety profile” of the FlowTriever system (Inari Medical) in 800 “real-world” patients. This is according to...
ClotTriever deemed a “game-changer” in deep vein thrombosis treatment
This advertorial, sponsored by Inari Medical, is only available in selected countries and geographies.
“I am finally very confident I have a device that...
Inari Medical announces randomised controlled trial evaluating clinical outcomes of the...
Inari Medical has announced planned enrolment of the DEFIANCE randomised controlled trial (RCT), which is designed to compare the clinical outcomes of patients with...
Inari Medical announces leadership succession plan
This week Inari Medical announced that chief operating officer Drew Hykes will succeed Bill Hoffman as chief executive officer, effective 1 January 2023. Hykes...
AVF 2022: Six-month CLOUT data indicate ClotTriever can effectively remove full...
Six-month outcomes from the ongoing CLOUT registry demonstrate the “safety and efficacy” of the ClotTriever thrombectomy system (Inari Medical) in a real-world deep vein...
First patient enrolled in PEERLESS study of FlowTriever system
Inari Medical has announced that the first patient has been enrolled in PEERLESS—a prospective, randomised controlled trial (RCT) comparing the outcomes of patients with...
Inari Medical announces six-month FLASH registry interim data
Inari Medical has announced positive acute and long-term interim results from the first 500 pulmonary embolism (PE) patients enrolled in the FlowTriever outcomes registry...
Registry data show greater clot chronicity on postprocedure inspection than initially...
Nearly half of patients deemed preprocedurally to be suffering an acute case of deep vein thrombosis (DVT) turned out to have a “much more”...
Inari Medical appoints pulmonologist Victor F Tapson as VP of Medical...
Inari Medical has announced that the company has appointed Victor F Tapson as vice president of Medical Affairs.
Tapson has devoted his medical career to...
CLOUT registry: Inari Medical announces positive interim results from first 250...
Inari Medical today announced "strongly positive" interim results from the first 250 deep vein thrombosis (DVT) patients enrolled in the CLOUT (ClotTriever outcomes) registry....
Inari Medical announces first patient enrolled in FLAME study
Inari Medical has announced the enrolment of the first high-risk pulmonary embolism (PE) patient in the FLAME (FlowTriever for acute massive pulmonary embolism) study....
Inari Medical announces presentation of positive chronic clot subanalysis results from...
Inari Medical has announced strongly positive interim results of the first 64 chronic deep vein thrombosis (DVT) patients enrolled in the ClotTriever outcomes (CLOUT)...
Inari Medical announces presentation of 30-day follow-up results from first patients...
Inari Medical has announced follow-up results of the first 230 patients enrolled in its FLASH study. FLASH is a real-world registry to study the...
FLASH registry: Interim results “reinforce the excellent FlowTriever safety profile”
Catalin Toma (University of Pittsburgh, Pittsburgh, USA) recently presented interim results from the FLASH registry during a late-breaking session at TCT Connect (14–18 October,...
Review of clot removal system shows positive short-term outcomes for lower...
Use of the ClotTreiver system (Inari Medical) for the treatment of lower extremity deep vein thrombosis (DVT) has demonstrated safety and efficacy, especially in...
First look at CLOUT registry reveals over 75% of DVT patients...
David J. Dexter, (Sentara Healthcare, Norfolk, VA, USA), principal investigator of the CLOUT registry using the ClotTriever Mechanical Thrombectomy System (Inari Medical) for treatment...
Inari Medical announces publication of FLARE IDE study results
Inari Medical has announced the publication of its 106-patient prospective multicentre FlowTriever Mechanical Pulmonary Embolectomy (FLARE) study for the treatment of intermediate-risk pulmonary embolism...
First patients enrolled in FLASH registry using the FlowTriever system for...
Inari Medical has announced the enrolment of the first patient in the FlowTriever All-Comer Registry for Patient Safety and Hemodynamics (FLASH) using the company’s...
Prospective clinical data on the FlowTriever system confirms excellent results in...
Inari Medical have announced the presentation of results from its FlowTriever Pulmonary Embolectomy (FLARE) clinical study that evaluated the safety and effectiveness of the...
Inari Medical announces completion of US$27 million Series C financing
Venture-backed medical device company Inari Medical has announced the close of a Series C financing totaling US$27 million. The company plans to use the capital to...
Inari Medical announces completion of enrolment in the FLARE study for...
Inari Medical has announced that it has completed enrolment of its investigational device exemption (IDE) study. The FlowTriever Pulmonary Embolectomy Clinical Study (FLARE) study...