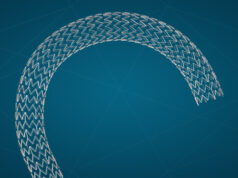
Gerard O’Sullivan, Department of Radiology, University Hospital Galway, Ireland, presented the results of the VIVO-EU prospective study of the Zilver Vena venous stent (Cook Medical) in the treatment of symptomatic iliofemoral outflow obstruction at the Cardiovascular and Interventional Radiological Society of Europe annual meeting (CIRSE; 10–14 September, Barcelona, Spain).
“I n a real-world population with multiple comorbidities, including patients with cancer and deep vein thrombosis, the patency rate for the Zilver Vena stent at one year is 89.7%. If you take out [from the study analysis] two patients of mine from Galway who had severe comorbidities, the stent patency at one year is 95%, which is fantastic. It is in line with the other trials coming out and I suspect that data from some of them will mirror these results,” O’Sullivan told Venous News.
VIVO-EU is a non-randomised study in Europe that set out to evaluate the Zilver Vena venous stent in the treatment of symptomatic iliofemoral venous outflow obstruction. It is the first large- scale prospective study in this space and there is also a US-arm currently underway. Zilver Vena was CE-marked in 2010 and is available in 14mm and 16mm diameters as well as 60, 100 and 140mm lengths.
In VIVO-EU, 35 patients with symptomatic iliofemoral outflow obstruction (as determined by CEAP ≥3 or a Venous Clinical Severity Score [VCSS] pain score of ≥2) were enrolled into the study with follow-up scheduled out to one year. Patients were treated as per usual medical practice. Nearly 41% of patients enrolled had acute deep vein thrombosis, 9% had acute on chronic deep vein thrombosis and 50% had chronic deep vein thrombosis. O’Sullivan is one of the principal investigators of the study along with Marta Ramirez Ortega (Madrid), Michael Lichtenberg (Arnsburg), Narayan Karunanithy (London) and Christoph Binkert (Switzerland).
“At one year, the rate of freedom from occlusion was 89.7% (29/33) correlated with improved venous clinical symptoms, suggesting that the stent is beneficial to patients. One year after treatment, mean Venous Quality of Life Questionnaire (CIVIQ) score went from over 50 to just under 30, Venous Disability Score improved following treatment and VCSS scores improved at all follow-up points after treatment,” he said.
Explaining the patency results further, O’Sullivan noted that in one patient, an unstable hip implant migrated into the pelvis and collapsed the stented vessel, preventing flow through the stent 20 days post-procedure. There was also occlusion or “no flow” documented in three other patients, all within 92 days of the study procedure.
There were 45 stents placed, an average of 1.3 per patient. The technical success, defined as successful delivery and deployment of the stent, was 97.8% (44/45 stents). Procedural success, defined as treated segment minimum lumen diameter ≥8mm at the end of the procedure and no major adverse events before discharge, was 97% (33/34 patients) as one patient experienced a pulmonary embolism before discharge. There were two major adverse events including a clinically-driven reintervention for occlusion at 155 days post-procedure (that was treated with thrombolysis, balloon angioplasty and an additional stent placement) and a procedure- or device-related pulmonary embolism that occurred one day post- procedure and categorised as possibly relating to the procedure (this was managed by a change in medication).
Laser-cut nitinol self- expanding stents will probably gradually replace Wallstent
Speaking to Venous News, O’Sullivan said: “While Wallstent (Boston Scientific) has the longest data to back its use in the treatment of deep vein thrombosis, there is already a clear shift to dedicated venous stents in that younger physicians feel more comfortable using laser-cut self-expanding nitinol stents. In the treatment of superficial femoral artery disease, for instance, very few patients would get a Wallstent nowadays—they would generally get a laser-cut nitinol self-expanding stent, whether it is drug- eluting or not. This shift is also likely to be seen in the venous sphere.” O’Sullivan also drew attention to the challenges in reimbursement faced by venous stenting in some European countries. “In countries such as Germany and France, there are significant challenges due to the lack of reimbursement for venous stenting. This is a huge impediment and might hamper venous therapies in these regions. In the UK and Ireland, which follow more of an American model, this is less of a problem. Deep venous disease is incredibly common and as awareness is growing, venous interventions are set to exponentially grow.
Venous interventions set to dramatically increase
O’Sullivan also drew attention to the results of the US National Institutes of Health (NIH)-sponsored ATTRACT (Acute venous thrombosis: thombus removal with adjunctive catheter- directed thrombolysis) trial, that are expected to be presented in early 2017. Its results could potentially support the use of additional catheter-directed thrombolysis in patients with extensive proximal deep vein thrombosis and pave the way for an explosion of venous interventions, including stenting. “I suspect that these results will change the game for quite some time to come,” he said.
“From looking at the number of people in the auditorium and in all the venous sessions at CIRSE, the interest in venous disease interventions continues to grow. Physicians are realising the benefit of stenting in post-thrombotic disease in chronically occluded venous segments. There are also other trials ongoing in the venous space such as Veniti with the Vici stent, Bard with their Venovo stent and the Vernacular trial and Medtronic have a stent called Abre, so this is an exploding space,” concluded O’Sullivan