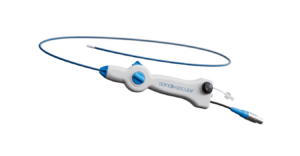
SonoVascular has announced the successful initiation of its first-in-human study (FIH) of the company’s SonoThrombectomy system, an ultrasound-facilitated, thrombolytic-enhanced platform that utilises multiple mechanisms of action to treat patients with venous thromboembolism (VTE). The catheter-based system combines microbubble-mediated cavitation with low-dose thrombolytic delivery to treat clots with no blood loss.
The FIH case was completed at the Hospital Dipreca in Santiago, Chile, under the supervision of vascular surgeon Manuel Espíndola, principal investigator (PI) at the site, with the assistance of Albrecht Krämer, also a vascular surgeon and the study PI. The clinical team successfully used SonoThrombectomy to treat deep vein thrombosis (DVT) involving the common and external iliac veins, SonoVascular reported in a press release.
“We were extremely pleased to perform the first clinical case using the SonoThrombectomy system,” said Espíndola. “The Resonator catheter performed exceptionally well, successfully and very quickly removing the thrombus and, most importantly, without the typical blood loss commonly seen in mechanical thrombectomy procedures. The patient was discharged from the hospital within 48 hours and is now at home, asymptomatic and recovering well.”
“The SonoThrombectomy system was effective at reducing thrombus burden while preserving vessel walls due to its unique and gentle approach,” added Krämer. “It is exciting that we achieved such a comprehensive result on the table with a very low dose of thrombolytic.”









