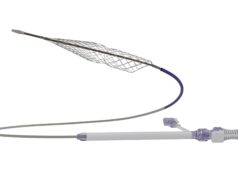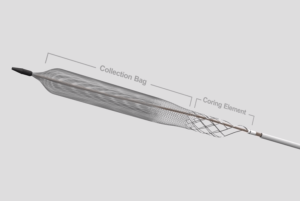
Inari Medical has announced that the first patient has been enrolled in DEFIANCE, a prospective randomised controlled trial (RCT) comparing the clinical outcomes of patients with iliofemoral deep vein thrombosis (DVT) treated with the ClotTriever system versus anticoagulation only.
The trial is led by principal investigators Xhorlina Marko (Beaumont Health, Dearborn, USA), Steven Abramowitz (MedStar Health, Washington DC/Leonardtown, USA), and Stephen Black (Guy’s and St Thomas’ Hospital, London, UK).
The first DEFIANCE patient was enrolled by Abdullah Shaikh (Allegheny Health Network, Pittsburgh, USA). DEFIANCE will enrol 300 DVT patients at up to 60 centres worldwide. DEFIANCE is Inari Medical’s second RCT, enrolling in parallel to the PEERLESS pulmonary embolism trial.
“We are thrilled to enrol the first patient and officially kick off this important clinical trial,” said Shaikh. “ClotTriever is fundamentally different to other DVT treatments. This trial is designed to produce definitive evidence to change standard of care.”
“DEFIANCE is an important step forward and will answer critical questions about how we manage DVT patients,” said Abramowitz. “Prior RCTs have focused on thrombolytic-based interventions, which have known downsides and limited effectiveness. This ClotTriever RCT is the first to compare lytic-free thrombectomy to anticoagulation for DVT.”
“With six major clinical studies, including two ongoing RCTs, Inari is wholly committed to [venous thromboembolism] patients and producing definitive evidence to support our technologies,” said Thomas Tu, chief medical officer of Inari Medical. “None of this is possible without the collaboration and dedication of our clinical trial investigators, who continually push the field forward in the name of better patient outcomes.”