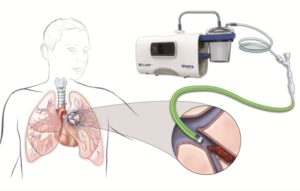
Insera Therapeutics’ CLEAR aspiration system has been used for the first time to treat a (human) patient with acute pulmonary embolism (PE). CLEAR, “cyclical luminal, evaluation, aspiration, and retrieval”, is a digital vacuum aspiration system that allows operators to employ uniform suction or cyclical suction patterns.
According to a press release, the “first-in-human success” of treating PE with CLEAR “comes on the heels” on the recently presented EXTRACT-PE trial results. Presented at the 2019 Vascular Interventional Advances (VIVA) meeting (4-7 November, Las Vegas, USA), the trial evaluated the safety and efficacy of the Indigo aspiration system.
In the study (119 patients), the system was associated with a significant mean reduction in right ventricular/left ventricular (LV) ratio of 0.43, corresponding to a 27.3% reduction, at 48 hours after intervention. The primary safety endpoint was reached with a low major adverse event composite rate of 1.7% within 48 hours. The Insera Therapeutics’ press release says this trial—despite it focusing on a different system—“advances treatment options for the industry as it establishes the use of vacuum aspiration in patients with acute PE”.
About the use of CLEAR, Luka Novosel (Sisters of Charity Hospital, Zagreb, Croatia) comments: “Due to the large clot burden in the lung arteries, the 65-year old gentleman presented with right-sided heart failure and was in critical condition. Traditional treatments using blood thinning medications such as anticoagulation and thrombolytic medications were contraindicated in this patient.
“We successfully treated this patient with massive bilateral acute pulmonary embolism using Insera’s CLEAR aspiration system. I am very pleased with the ease of use of cyclical aspiration and its ability to rapidly ingest large amounts of clot. It also provides a safer mechanical means of removing life-threatening blood clots without the bleeding risk associated with blood thinning medications.”
The CLEAR aspiration system has European regulatory approval for the aspiration of blood clots, and is indicated for use for acute ischaemic stroke secondary to large vessel occlusive disease. It also has CE mark approval for aspiration of blood clots from arteries (e.g. PAD, PE, and coronary artery disease) and for the aspiration of blood clots from veins (in patients with deep venous thrombosis, cerebral venous sinus thrombosis or patients with renal failure who have clotted haemodialysis grafts).
Vikram Janardhan, CEO of Insera Therapeutics, comments: “There is a huge unmet need for innovative devices for the removal of life-threatening blood clots. Our cyclical aspiration technology has been shown to improve first-pass recanalisation, reduce distal embolisation, and has an improved safety profile at the time of the procedure. In the USA, it is estimated that every year there are around 225,000 patients with ischaemic strokes due to large vessel occlusions, 300,000 patients with PE, 600,000 patients with deep vein thrombosis, and 100 patients with acute limb ischaemia.”