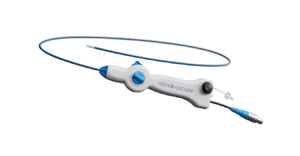
SonoVascular has announced the successful completion of an initial set of eight deep vein thrombosis (DVT) cases using its SonoThrombectomy system as part of a first-in-human (FIH) study of the device.
All cases achieved a complete Marder score reduction as assessed by an independent core laboratory, while significant improvements from baseline in Villalta score, revised Venous Clinical Severity Score (rVCSS) and Numeric Pain Rating Scale scores were observed at 30-day follow-up, the company reported. There were no device-related adverse events, major bleeding, or deaths.
The data were recently shared during the 2025 American Venous Forum (AVF) in Atlanta, Georgia (Feb. 16–19) by William Marston (University of North Carolina at Chapel Hill, USA).
“The results obtained thus far in SonoVascular’s FIH study demonstrate the SonoThrombectomy system has the potential to become a disruptive technology that would effectively overcome the shortcomings of available therapies for the treatment of DVT,” he said.
“In the initial cases, the SonoThrombectomy system has demonstrated its ability to eliminate intravenous clot in a single treatment session with no blood loss and with preservation of venous valves. The system is not limited by the presence of previously implanted filters or stents and is highly steerable for treatment of specific areas of residual thrombus.”
The SonoThrombectomy system—designed for the treatment of venous thromboembolism (VTE)—delivers ultrasound energy and microbubbles directly to clots through the Resonator catheter, inducing microbubble-mediated cavitation, which mechanically breaks down clots, according to SonoVascular. Additionally, a very low dose of tissue plasminogen activator (tPA) is infused in combination with the microbubbles through the catheter to further improve clot breakdown.









