A new study presented during the 2024 American Venous Forum (AVF) in Tampa, Florida (March 3–6), that compared a novel non-pneumatic compression device with an advanced pneumatic compression modality in ther treatment of lower extremity lymphedema demonstrated “statistically significant disruption” in limb volume that favored the former.
The multicenter, randomized controlled crossover study’s primary endpoint was change in limb volume from baseline, change in quality of life using the LYMQOL validated quality-of-life scoring system, and treatment adherence during the study period, presenter Todd Berland, MD, director of outpatient vascular interventions at NYU Langone Health in New York, New York, told those gathered in a late breaking session at AVF 2024.
The patients in the study had an average of 58 years old, and a majority of them were White women. Of the cohort, there were 11 with primary and 60 with secondary lymphedema, 34 were unilateral and 37 bilateral, and they had an average of eight years since diagnosis.
Berland outlined the non-pneumatic technology at hand: the Dayspring (Koya Medical) device, a battery-powered without a foot portion, which allows users to walk, features components that “stretch and release, and fake or simulate a manual lymphatic massage experience,” he said. “Between the static compression that you get with the actual garment,” Berland continued, “the stretching and elongating phenomenon—and the fact that you can walk with the device, and really activate that calf muscle pump—may account for what we see here in this study, [where] we compare this device to commercially available advanced pneumatic plug-in-the-wall devices.”
“We had nine study sites with 121 patients consented,” he told AVF 2024. “There were 22 screening failures, leaving us with 99 patients.”
The team randomized patients to the non-pneumatic or advanced compression device, which patients then used for three months. “At the end of that three-month period, we checked quality-of-life and limb-volume measurements,” Berland said. “We then had them use nothing for one month, the washout period, to give us a new baseline. We rechecked limb volume and quality-of-life, and then the patients crossed over.”
After cross over, they used the opposite device that they were originally given for another three-month period. Measurements were taken of the patients’ lower extremities. Quality of life was also taken at the end of three months. Finally, patients were given a diary to record their thoughts of the devices throughout the study.
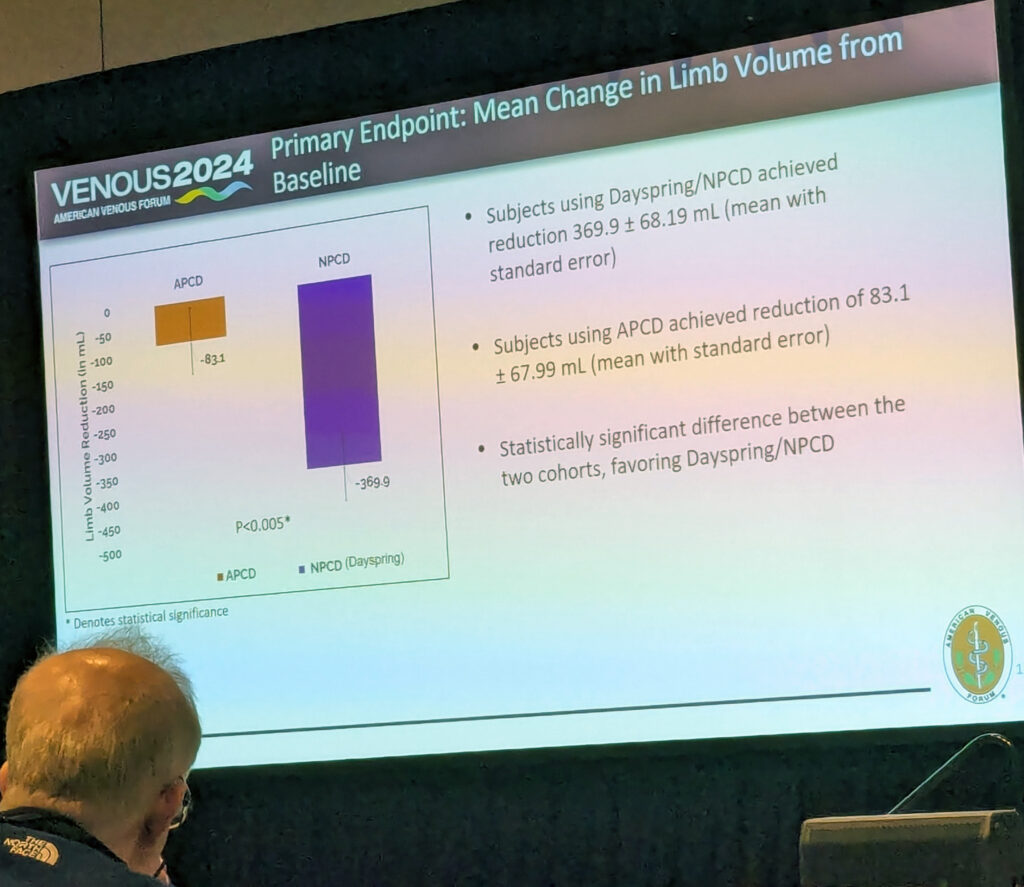
Results showed a “statistically significant disruption in limb volume favoring the Dayspring device,” Berland told the audience. “A 370mm volume reduction in this group compared to an 83mm reduction in advanced pneumatic compression.”
“When we look at the foot, there was actually no statistical significant difference here. Both devices and subgroups trended toward improved diameters and circumference of the foot,” he said.
The quality-of-life questionnaire also favored the Dayspring device, with the function, appearance and symptoms being rated as better than the advanced pneumatic compression device. Even when it came to how committed the patients in the study were, the Dayspring device had an adherence rate of 81% compared to the advanced pneumatic compression rate of 56%, Berland added.
Finally, when it came down to which of the two devices the patients preferred, “78% of patients preferred the Dayspring device compared to 22% for advanced pneumatic compression,” Berland said.









