Thrombolex recently announced the publication of RESCUE-II study results in JACC: Advances.
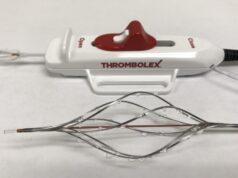
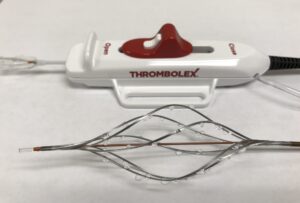
The RESCUE-II study was a single-centre, prospective study evaluating the safety and feasibility of on-the-table (OTT) pharmacomechanical lysis (PML) without postprocedural infusion when treating patients with acute intermediate-risk pulmonary embolism (PE). Nine patients were enrolled and successfully treated with low dose r-tPA (4mg per pulmonary artery) using the Bashir endovascular catheter (BEC).
Thrombolex reports that, at 48 hours, the mean right ventricle to left ventricle (RV/LV) ratio decreased by 22.3%, and pulmonary artery obstruction, as measured by the Refined Modified Miller Index, was reduced by 29.2%. There were no major bleeding events, no deaths or serious adverse events through 30-day follow-up.
“This marks a significant milestone for our company, as we are proud to contribute high-quality evidence to the field and remain committed to advancing innovative treatments,” said Michael Cerminaro, president and chief executive officer of Thrombolex. “We would like to thank Senator Steve Santarsiero and the Department of Community and Economic Development of the Commonwealth of PA for their grant funding support of this important clinical research.”
“The RESCUE-II study demonstrated encouraging safety and feasibility, while reinforcing the clinical value of this novel OTT protocol,” said, Vlad Lakhter (Temple University Hospital, Philadelphia, USA). “These results warrant further investigation in larger, multicentre trials like the RAPID-PE study, which is currently enrolling patients.”
Thrombolex advises that an independent data safety monitoring board adjudicated all clinical events for the RESCUE-II study, while imaging data were assessed by an independent Core laboratory.