Tag: thrombolex
RESCUE II results on Thrombolex’s Bashir endovascular catheter for PE published
Thrombolex recently announced the publication of RESCUE-II study results in JACC: Advances.
The RESCUE-II study was a single-centre, prospective study evaluating the safety and...
Thrombolex begins enrolment in RAPID-PE study of Bashir catheter
Thrombolex has announced the enrolment of the first two patients in the RAPID-PE study using the Bashir endovascular catheter for the treatment of acute...
Thrombolex and Aidoc announce strategic partnership to advance breakthrough PE treatment
Thrombolex and Aidoc have announced a strategic partnership aimed at revolutionising the treatment of acute pulmonary embolism (PE), a press release reports.
This strategic collaboration,...
Thrombolex announces new insights from the RESCUE trial with the Bashir...
Thrombolex has announced never-before-reported major reductions in obstruction in all of the segmental pulmonary arteries (PA), based on independent core lab data analysis of...
RESCUE trial shows reduction in segmental and main pulmonary artery occlusions
Robert A Lookstein, who is executive vice chair, Diagnostic, Molecular and Interventional Radiology at the Icahn School of Medicine at Mount Sinai Hospital (New...
RESCUE trial of the Bashir endovascular catheter demonstrates reduction in pulmonary...
Thrombolex today presented the final results of its National Institutes of Health (NIH)-sponsored RESCUE trial at TCT 2022 (16–19 September, Boston, USA).
This investigational...
Michael Cerminaro to succeed Marvin Woodall as CEO of Thrombolex
Thrombolex has announced that its board of directors has named Michael Cerminaro chief executive officer (CEO) effective 13 July 2022, the date of the...
Thrombolex announces new interim data from RESCUE trial of the Bashir...
Thrombolex has announced positive results from the prespecified interim analysis of the first 62 evaluable pulmonary embolism (PE) patients enrolled in the investigational RESCUE...
Thrombolex announces interim RESCUE results at VIVA 2021
Thrombolex recently announced the results of the RESCUE trial’s prespecified interim analysis, of the first 62 evaluable patients, in a late-breaking clinical trials session...
Thrombolex receives enhanced indication for use from the FDA
The US Food and Drug Administration (FDA) has enhanced the indications for use of the Bashir endovascular catheter (Thrombolex) and the Bashir Plus endovascular...
First-in-human results for the Bashir endovascular catheter published
Results of a first-in-human study to assess the safety and feasibility of the Bashir endovascular catheter (Thrombolex) for the treatment of acute intermediate-risk pulmonary...
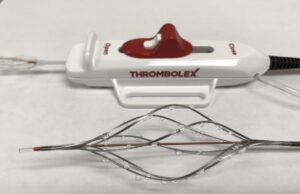
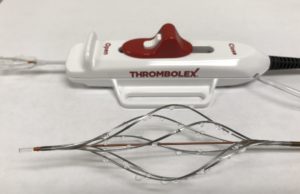
The BASHIR™ Endovascular Catheter: Delivering a complete solution for arterial and...
The BASHIR™ Endovascular Catheter offers a safe and easy-to-use platform technology, creating immediate blood flow, accelerating thrombolysis, and dissolving large volumes of thrombus burden....
First patient enrolled in RESCUE trial of Thrombolex’s Bashir catheter
Thrombolex has announced that it has enrolled the first patient in its pivotal RESCUE trial for the treatment of patients with acute submassive pulmonary...
Thrombolex receives NIH grant to fund RESCUE trial of Bashir endovascular...
Thrombolex has announced that it was awarded a US$3 million Small Business Innovation Research (SBIR) grant from the National Heart, Lung and Blood Institute...
First-in-human results for Bashir Endovascular Catheter meet safety and feasibility endpoints
Thrombolex has announced that results of its First-In-Human (FIH) trial confirm the early safety and feasibility of using the Bashir Endovascular Catheter for pharmacomechanical...
FDA approval granted to Thrombolex for Bashir Endovascular Catheter-Short Basket
Thrombolex has announced that the company has received US Food and Drug Administration (FDA) clearance for the Bashir Endovascular Catheter - Short Basket (BEC...
Thrombolex announces first enrolment in early feasibility and safety study using...
Thrombolex has announced the enrolment of the first patient in their Early Feasibility and Safety Study, investigating the Bashir Endovascular Catheter for the treatment...
Thrombolex announces 510(k) clearance of Bashir catheter systems for thromboembolic disorders
Thrombolex has announced that the US Food and Drug Administration (FDA) has cleared the Bashir Endovascular Catheter (BEC) for the controlled and selective infusion...