Tag: BTG
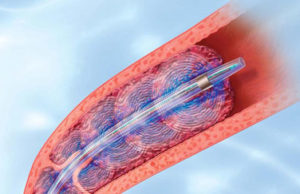
First successful implantation of the bioconvertible Sentry IVC filter
The world’s first bioconvertible IVC filter commercially offered in the USA (Sentry; from BTG) has been successfully implanted into the first patients outside of...
Boston Scientific announces recommended offer to acquire BTG
Boston Scientific has announced it has reached an agreement on the terms of a recommended offer to acquire BTG, a company headquartered in the...
Sentry Bioconvertible IVC filter two-year results show zero tilt or migration
Two-year results for the SENTRY trial were presented at the Vascular Interventional Advances conference (VIVA; 5–8 November, Las Vegas, USA). The prospective, multicentre trial...
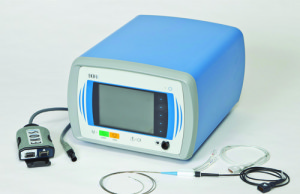
BTG to begin European distribution of EKOS Control unit 4.0
BTG has announced that the first EKOS Control Unit 4.0 have been shipped from BTG’s facility in Bothell (Washington, USA) to Europe, where full...
BTG acquires Novate Medical
BTG has announced it has acquired Novate Medical, a medical device company focused on the prevention of pulmonary embolism in patients at high risk...
PERT Consortium and BTG form strategic partnership
BTG has announced a strategic partnership with the PERT Consortium to advance the science of pulmonary embolism treatment and promote the implementation of PERT...
Non-thermal techniques for treatment of venous ulcers: Off-label use of cyanoacrylate...
At the American Venous Forum (AVF; 20–23 February, Tucson, USA), a session on venous leg ulcers turned to possible future treatment strategies as Kathleen...
Study to measure shorter duration EKOS therapy for pulmonary embolism is...
Global specialist healthcare company BTG has highlighted the commencement of the KNOCOUT PE study. The KNOCOUT PE study will measure how hospitals and patients...
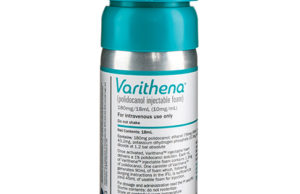
BTG receives finalised category I CPT codes for Varithena
BTG has announced its finalised category I CPT codes, received from the US Centers for Medicare and Medicaid Services. The codes will be be...
ACCESS PTS study demonstrates efficacy of EKOS therapy for post-thrombotic syndrome
The results of the ACCESS PTS trial have been presented at the Society for Vascular Medicine 28th Annual Scientific Sessions (14–17 June, New Orleans,...
OPTALYSE PE study demonstrates safety and efficacy of shorter, lower dose...
Results from the OPTALYSE PE trial have been presented at the American Thoracic Society International Conference in Washington, DC, USA. The results show that...
BTG’s Varithena launched in Canada
Following Health Canada regulatory approval, BTG is to launch its polidocanol injectable foam (Varithena), a drug/device combination product used to treat varicose veins, in...
First pulmonary embolism patients in Hong Kong treated with BTG’s EKOS...
The first Hong Kong-based patients diagnosed with pulmonary embolism have been treated using the newly available BTG EKOS system. The EKOS system includes an...
Varithena earns FDA approval for 30-day post-activation shelf life
BTG has announced that the US Food and Drug Administration (FDA) has approved an extension of the post-activation shelf life of Varithena (polidocanol injectable foam)...