Tag: CVI
Basis Medical announces successful first-in-human procedures using Seclusion catheter
Basis Medical has announced the successful completion of its first-in-human clinical procedures using the Seclusion catheter to treat chronic venous insufficiency (CVI) caused by...
Basis Medical’s Seclusion catheter for superficial vein reflux receives US FDA...
Basis Medical recently announced that its Seclusion catheter for superficial vein reflux has received US Food and Drug Administration (FDA) 510(k) clearance.
The company notes...
One-year data from the VenoValve US pivotal trial emerge
Envveno Medical has announced that it will present one-year data on all patients from the VenoValve US pivotal trial today at the VEITHsymposium (19–23...
InterVene announces US$13 million Series A financing to advance Recana system...
InterVene recently announced the closing of its US$13 million Series A financing round. The financing was co-led by new investor Treo Ventures and existing...
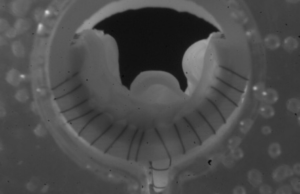
Prosthetic valve implantation shows “vast” improvements in deep chronic venous insufficiency
“The surgical challenges of repairing deep venous reflux have been present for more than a generation. It is worth reflecting on the fact that...
Cook Medical treats first patient in first-in-human clinical trial for venous...
Cook Medical recently announced that the first patient has been treated in a clinical study to evaluate a new venous valve designed for treating...
First-in-human patients continue to benefit from VenoValve at average of three...
Positive long-term, three-year observational data from a cohort of patients that participated in the previously concluded VenoValve (Envveno Medical) first-in-human clinical trial were recently...
I-Vasc announces €1.8 million Series A investment to launch its Velex...
I-Vasc, developer of the Velex device with its empty vein ablation (EVA) technology for the treatment of chronic venous insufficiency (CVI), has announced the...
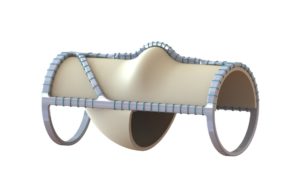
AVF 2022: Emerging autogenous venous valve formation system sees “continual improvement”
An emerging endovenous valve formation system designed to treat patients with chronic venous insufficiency (CVI) with evidence of deep venous reflux has demonstrated continual...