Tag: Envveno Medical
enVVeno receives ‘unfavorable’ appeal decision from FDA for VenoValve device
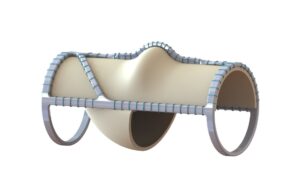
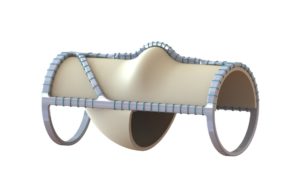
enVVeno Medical has announced that it has received an "unfavorable" decision from the US Food & Drug Administration (FDA) in response to its supervisory...
Appeal filed over ‘not-approvable’ letter for breakthrough venous valve replacement
Venous valve developer enVVeno Medical has announced that it will file a request for supervisory appeal of the "not-approvable" letter from the Center for...
US FDA deems Envveno’s VenoValve “not-approvable”
The US Food and Drug Administration (FDA) has issued a letter to Envveno Medical stating that its VenoValve technology is "not-approvable," a company press...
Three-year VenoValve first-in-human trial data published
Envveno Medical has today announced that its manuscript titled, ‘Three-year outcomes of surgical implantation of a novel bioprosthetic valve for the treatment of deep...
VenoValve: Analysis finds similar improvement among both primary and thrombotic deep...
A new subanalysis of the SAVVE (Surgical antireflux venous valve endoprosthesis) trial found that there was no difference in the level of improvement in...
One-year data from VenoValve US pivotal trial highlighting impact on patients’...
One-year follow-up data on 75 subjects from the VenoValve (Envveno Medical) US pivotal trial were presented by principal investigator Cassius Iyad Ochoa Chaar (Yale...
One-year data from the VenoValve US pivotal trial emerge
Envveno Medical has announced that it will present one-year data on all patients from the VenoValve US pivotal trial today at the VEITHsymposium (19–23...
PMA application submitted for VenoValve device
enVVeno Medical today announced it has submitted an application with the U.S. Food and Drug Administration (FDA) seeking approval to market the VenoValve—a surgical...
Deep venous valve technologies set to address “large unmet need worldwide”
The pursuit of a cure for deep venous valvular reflux—long considered to be the “holy grail” of deep venous disease—is underway, with new technologies...
Venous valve technologies set to address “large unmet need worldwide”
“I see a future where this technology may be used to treat people early and avoid the devastating long term consequences of venous insufficiency”...
First-in-human patients continue to benefit from VenoValve at average of three...
Positive long-term, three-year observational data from a cohort of patients that participated in the previously concluded VenoValve (Envveno Medical) first-in-human clinical trial were recently...