Raghu Motaganahalli (Indiana University School of Medicine, Indianapollis, USA) discusses the expanding space of surgical treatment of severe chronic venous insufficiency (CVI), novel technologies that have emerged to address challenges and the data which may direct a pathway to best patient care.
Severe CVI results from valvular incompetence in the deep veins of the leg. While CVI resulting from superficial venous insufficiency can be effectively treated through surgical ablation of the saphenous vein, vascular surgeons have limited options for treating patients with CVI of the deep venous system. Although some patients with CVI of the deep veins may be candidates for valvuloplasty, few undergo the procedure, both because it requires the presence of a repairable valve and because it is a complex procedure performed by a limited number of vascular surgeons and with inconsistent results. As such, it is not a viable treatment option for the vast majority of patients with severe deep venous CVI.
The absence of a standardised and effective surgical approach to treating severe CVI limits vascular surgeons’ ability to help improve the lives of the estimated 2.5 million people in the USA living with this serious and debilitating condition. Without effective therapy, these patients are counseled to wear compression garments and elevate their legs—which do not address the underlying cause of disease and provide limited benefits in managing symptoms—as their quality of life remains compromised and their risk of developing venous ulcers remains unmitigated.
An evolving landscape for surgical treatment of severe deep venous CVI
A key limitation of valvuloplasty is the need for a repairable native valve, which is often absent in patients with severe deep venous CVI. Even in eligible patients, the procedure is technically complex and requires long-term experience and skill. Bioprosthetic venous valve technology is now poised to overcome this challenge, offering the potential for an off-the-shelf device that restores the function of multiple damaged valves in the deep veins of the leg. Like the bioprosthetic and mechanical valves used for decades in structural heart disease indications, a surgical replacement venous valve made from porcine tissue is currently under review by the US Food and Drug Administration (FDA) for approval.
This bioprosthetic venous valve is implanted in the femoral vein, in the upper mid-thigh area via the surgical antireflux venous valve endoprosthesis (SAVVE) procedure, a novel open surgical approach. Once implanted, it functions as a one-way valve, restoring proper directional blood flow back to the heart, and providing optimal haemodynamics, minimising flow disturbances and reducing the risk of thrombus formation. In clinical trials conducted to date, patients undergoing the SAVVE surgical procedure typically remain in the hospital for 23 hours post procedure. Patients receive anticoagulant therapy for at least one year following the procedure, but may require longer-term anticoagulation, typical of that seen in patients who receive mechanical heart valves.
The FDA’s decision on this innovative surgical replacement venous valve, anticipated in the second half of 2025, could provide vascular surgeons with a promising and novel treatment option for patients with severe deep venous CVI caused by valvular incompetence of the deep venous system.
US trial data support the efficacy and safety of the SAVVE procedure
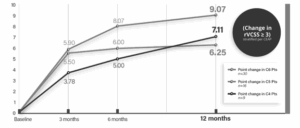
The potential for this new bioprosthetic venous valve to enable a standardised, surgical approach to correcting the underlying valvular incompetence that causes severe CVI of the deep veins was a key motivator for my participation as a principal investigator in the VenoValve US pivotal trial. This trial was performed at 21 US sites and included 75 patients with CEAP classifications C4b (6.8%), C4c (6.8%), C5 (28.8%), and C6 (57.5%). The mean age of trial participants was 62.9 years (range 38–83 years). I believe the results of this trial are very compelling. At 12 months post procedure, 85% of participants achieved a clinically meaningful benefit, which was defined as at least a three-point improvement in the revised Venous Clinical Severity Score (rVCSS).
Additionally, the median percent reduction in ulcer area (cm2) was 87% across all trial participants. This included nearly complete healing of ulcers that had persisted for three to 12 months prior to study entry and, notably, substantial healing of ulcers that had persisted for more than a year.
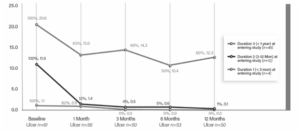
Improvements in multiple patient-reported outcomes also support the benefits of the SAVVE procedure, including a 74.8% median reduction in Visual Analog Scale (VAS) target leg pain scores, a 91.9% mean improvement in Venous Issufficiency Epidemiological and Economic Study (VEINES) symptom scores, and a 57.8% improvement in VEINES quality-of-life scores.
The pivotal trial also showed excellent procedural outcomes, with a 97.3% success rate and 100% intraoperative device patency. At 12 months post procedure, patency rates were 98.4% for the device and 96.8% for the target vein.
No pulmonary embolisms occurred in the trial and there was one death, which was unrelated to the bioprosthetic valve. Major adverse events (MAEs) were 14 target vein thromboses, 10 surgical pocket haematomas, four other bleeds, and eight deep wound infections. It’s important to note that 94% of the subjects who experienced an MAE (not including the unrelated death) also experienced a clinical meaningful benefit.
Integrating bioprosthetic venous valve technology into clinical practice
While new surgical procedures typically take time to learn, a key advantage of the new bioprosthetic venous valve is the short learning curve for the SAVVE surgical procedure. Vascular surgeons trained in open vascular surgery techniques will have the surgical skills required for the SAVVE procedure. The minimal learning curve for this innovative surgical approach should enable its rapid adoption, which is essential for making the SAVVE procedure broadly available to patients who may benefit from it. I also am highly encouraged by the subset analyses demonstrating clinically meaningful benefit across multiple CEAP categories (C4b–C6), which should help guide patient selection for the SAVVE procedure. I believe that the pending FDA approval of the SAVVE procedure is an important advance for the vascular surgery and severe CVI patient communities, and I look forward to finally have the tools I need to help improve the lives of my patients living with severe CVI of the deep veins.
Raghu Motaganahalli is a professor of surgery and division chief and programme director for vascular surgery at the Indiana University School of Medicine, Indianapolis, USA.
Disclosures
Raghu Motaganahalli was principal investigator for the SAVVE trial.