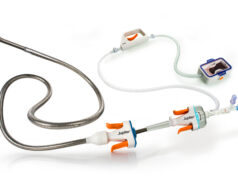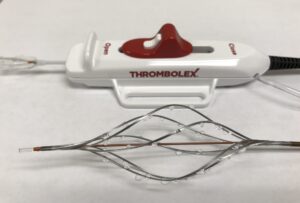
Thrombolex has announced the enrolment of the first two patients in the RAPID-PE study using the Bashir endovascular catheter for the treatment of acute pulmonary embolism (PE). The patients were enrolled by Ayman Iskander (St Joseph’s Health Hospital, Syracuse, USA).
RAPID-PE is a single-arm, multicentre, postmarket study evaluating the safety and effectiveness of on-the-table pharmacomechanical lysis (PML) without post-procedural thrombolytic infusion using the new 0.035” guidewire-compatible Bashir endovascular catheter. “This next-generation platform technology is engineered for optimal resolution of thrombus in the treatment of PE. Patients with acute intermediate-risk PE are treated with four 1mg pulse sprays of r-tPA [recombinant tissue plasminogen activator] into each pulmonary artery without subsequent r-tPA infusion,” a press release reads. “The first two patients treated were discharged from the hospital in good health less than 24 hours post procedure.”
Iskander commented: “It was exciting to see a dramatic improvement in haemodynamics on the table in both patients with very short treatment times (one bilateral; 38 minutes and one unilateral; 16 minutes) in the cath lab. This approach may eliminate the need for an ICU [intensive care unit] stay, which could be a boon to the overburdened health systems.”
Wissam Jaber (Emory University, Atlanta, USA), co-national principal investigator of the RAPID-PE study, commented: “We are pleased to see the enrolment of the first two patients in the RAPID-PE study and are very encouraged by the clinical response with this novel PML approach. We believe this protocol will be as effective as traditional PE therapies and potentially safer, due to the ultra-low dose of lytics used. This is a logical next step in improving upon the excellent clinical results from the original RESCUE study.”
The RESCUE study enrolled 109 patients with intermediate-risk PE at 18 US hospitals and “showed unsurpassed effectiveness and safety in this patient population,” according to Thrombolex. The data from a related single-centre study from Temple University Hospital, the RESCUE-II study, that uses the on-the-table protocol will be presented at TCT (27–30 October, Washington DC, USA).