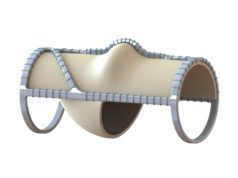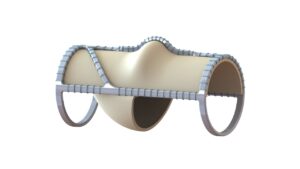
enVVeno Medical has announced that it has received an “unfavorable” decision from the US Food & Drug Administration (FDA) in response to its supervisory appeal of the not-approvable letter it received on 19 August in response to its premarket approval (PMA) application for the VenoValve device, a surgical replacement venous valve for treating severe deep chronic venous insufficiency (CVI).
The supervisory appeal upheld the review staff decision in the not-approvable letter that the VenoValve did not meet the standard of reasonable assurance of safety and effectiveness, enVVeno reported.
“Although the appeal decision was not the result we are looking for, it did provide valuable insight into the criteria that would be necessary for approval of enVVe, our next generation transcatheter based replacement venous valve,” said Robert Berman, enVVeno Medical’s CEO. “enVVe should have a different safety profile than an open surgical device and is ready for human testing. Assuming that we can reach alignment with the Agency on achieveble endpoints for enVVe, it makes sense to turn our attention and devote our resources to enVVe. We will continue to interact with the FDA on enVVe and will provide periodic updates on our progress.”