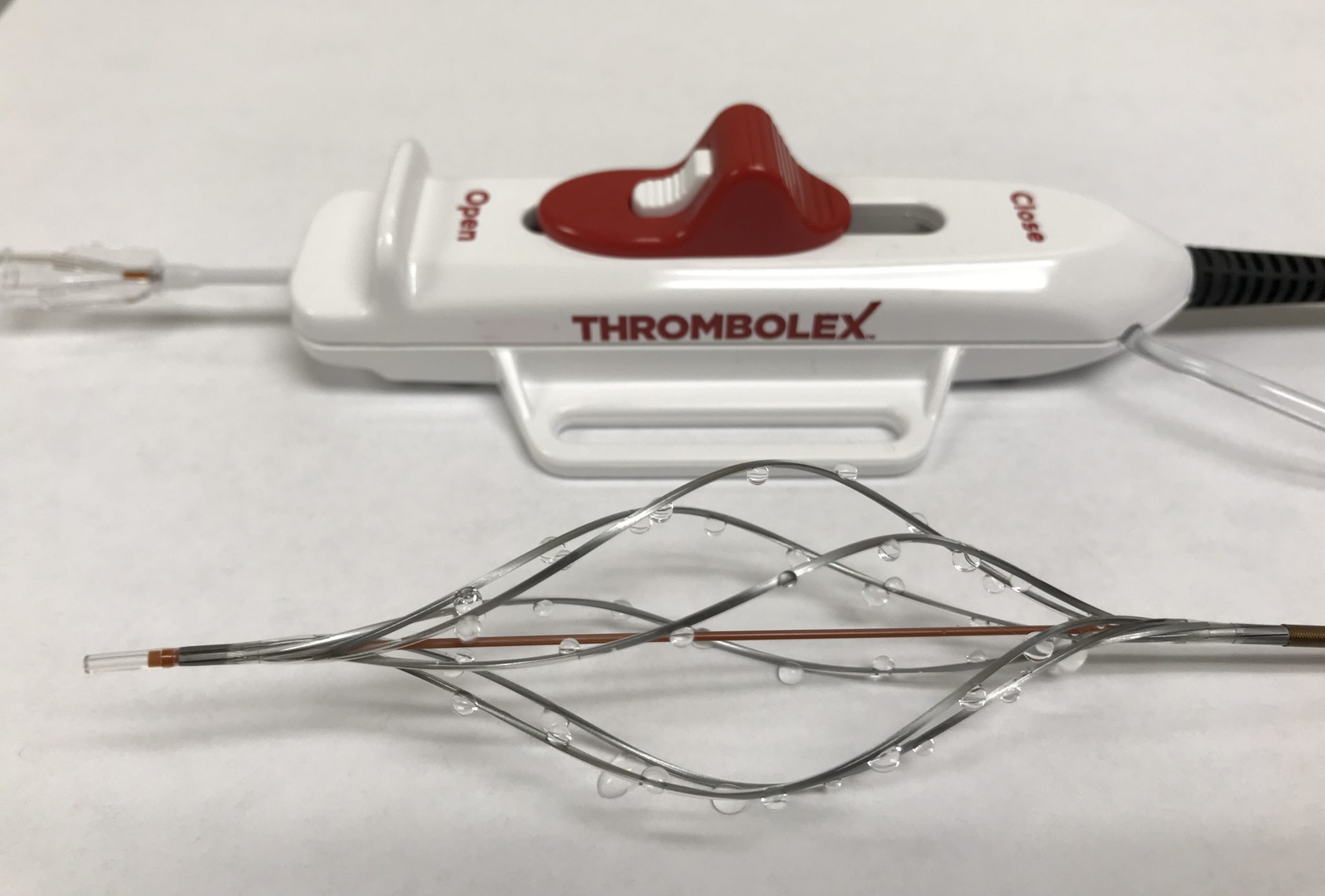

Thrombolex has announced that it has enrolled the first patient in its pivotal RESCUE trial for the treatment of patients with acute submassive pulmonary embolism (PE) using the Bashir endovascular catheter, under an Investigational Device Exemption (IDE) from the US Food and Drug Administration (FDA).
RESCUE is a prospective, single-arm, multicentre trial of pharmaco-mechanical catheter-directed thrombolysis (CDT) and will enrol up to 125 patients at up to 20 sites in the USA.
The first patient was a 50-year-old man with a high-risk submassive pulmonary embolus affecting both lungs, treated at Charleston Area Medical Center, Vascular Center of Excellence, Clinical Trials Center and WVU Vascular Surgery Center, Charleston, USA by Aravinda Nanjundappa.
“The patient had an excellent result with no complications following treatment with two Bashir endovascular catheters, five hours of infusion and a total of 14mg of r-tPA being administered. We were privileged to enrol the first patient in this important PE trial using the Bashir endovascular catheter,” said Nanjundappa.
The RESCUE study follows on the very successful first-in-human (FIH) trial completed by Thrombolex at the end of last year. The FIH trial met its primary safety and feasibility endpoints, and showed large and rapid reduction in right heart strain as evidenced by a reduction in RV/LV ratio of 37% (p<0.0009), as well as a 37.1% mean reduction (p< 0.0005) in pulmonary clot burden, as measured by the Modified Miller Index (MMI) following infusion of 14mg of r-tPA over 8 hours.
There were no major bleeding or other adverse events. “Based on the impressive early results of the FIH study I am excited to see a larger set of real world data using the Bashir endovascular catheter,” said Akhilesh Sista, section chief of Vascular & Interventional Radiology at NYU Langone Health and member of the Steering Committee for RESCUE.
The Bashir endovascular catheter is a pharmaco-mechanical CDT device that received 510(k) premarket regulatory clearance for the controlled and selective infusion of physician-specified fluids, including thrombolytics, into the peripheral vasculature. The goal of RESCUE is to achieve an additional indication for its use in the treatment of PE.