Venture-backed medical device company Inari Medical has announced the close of a Series C financing totaling US$27 million. The company plans to use the capital to commercialise its catheter devices for the treatment of venous thromboembolism.
The financing was led by new investor Gilde Healthcare and was joined by all of Inari’s existing investors including Versant Ventures and US Venture Partners. Geoff Pardo of Gilde Healthcare will join Inari’s Board of Directors.
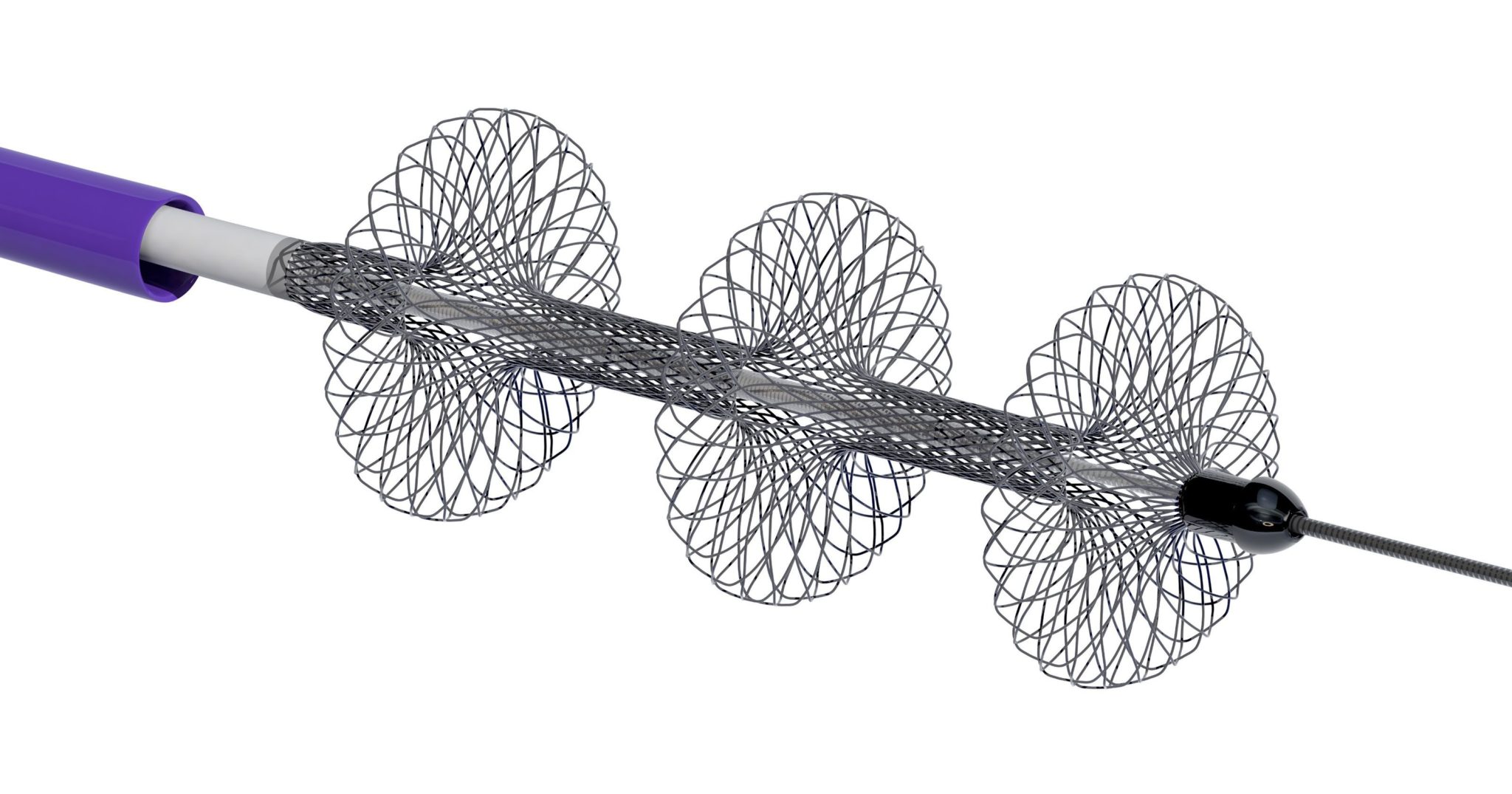
The financing provides Inari with capital to commercialise its catheter-based technologies for the treatment of venous thromboembolism (VTE). To date, Inari has developed the FlowTriever and ClotTriever Systems, both of which are designed to enable the safe removal of large clot volumes from big vessels without the use of thrombolytic drugs.
“We are fortunate to have the support of a strong consortium of life sciences venture capital firms and would like to welcome both Gilde Healthcare as an investor in Inari and Geoff Pardo as a Board member,” said Bill Hoffman, President and Chief Executive Officer. “We will use these funds to support and accelerate our commercial, clinical and technology development programs.”
“There is a significant need to improve outcomes for the millions of patients suffering from VTE,” added Geoff Pardo of Gilde Healthcare. “We have been impressed by the Inari team, the progress they’ve made to date and their vision to transform the treatment of VTE.”
Inari Medical is a privately held venture backed medical device company specialising in the development of innovative catheter-based technologies for the treatment of venous thromboembolism. Inari is focused on solutions that enable the safe removal of large clot volumes from big vessels without the use of thrombolytic drugs. The company has developed two novel mechanical thrombectomy technology platforms, the FlowTriever and ClotTriever Systems. Both Systems are 510(k) cleared by the US FDA for thrombectomy in the peripheral vessels and have also received the CE mark. Inari was founded in 2013 as a spin-out of Inceptus Medical, a medical device incubator. The company is backed by Gilde Healthcare, Versant Ventures, US Venture Partners, the founders, and other private investors.









