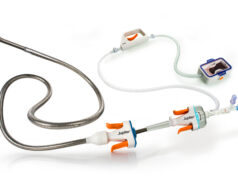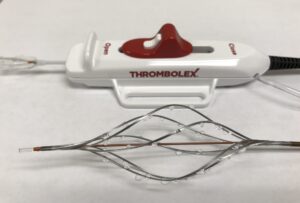
Prespecified interim analysis data from the first 50 patients in RAPID-PE have demonstrated “excellent safety” and “remarkably efficient lab times” in patients receiving on-the-table pharmacomechanical lysis without any post-procedure infusion with the Bashir endovascular catheter (Thrombolex) for the treatment of intermediate-risk acute pulmonary embolism (PE). The findings were presented during the 2025 Transcatheter Cardiovascular Therapeutics (TCT) conference (25–28 October, San Francisco, USA).
RAPID-PE, a single-arm, prospective, multicentre US study, protocol delivers 4mg recombinant tissue plasminogen activator (r-tPA) into each pulmonary artery (8mg total for bilateral PE) by multiple hand-injected pulse sprays using an expanded 8Fr device. The dual mechanism of action is designed to create immediate mechanical fissures to restore blood flow while enabling low-dose lytic to act within and beyond the clot.
Interim data for the primary endpoint showed no mortality or hemodynamic decompensation at seven days. Major bleeding was seen in 2% and the median total procedure time was 43.5 minutes, with a median catheter dwell time of 17.5 minutes. Eight percent of patients went to after the procedure and the median length of hospital stay was 2.9 days. These procedures were performed by 17 different operators across various participating sites.
Presenting author Wissam Jaber (Atlanta, USA), RAPID-PE co-national principal investigator, commented: “This interim cohort demonstrates that [the device] can democratize the catheter-based treatment of intermediate-risk PE patients with excellent safety and remarkably efficient lab times, often without the need for an ICU stay.”