Tag: FDA
FDA begins “real-time” reporting of adverse event data
The US Food and Drug Administration (FDA) recently announced that it has begun daily publication of adverse event data from the FDA Adverse Event...
US FDA announces plans to address medical device shortage risks
The US Food and Drug Administration (FDA) has issued a statement outlining measures to enhance protections against medical device shortages.
According to Michelle Tarver,...
US FDA issues first draft guidance for AI-based medical device development
The US Food and Drug Administration (FDA) has issued draft guidance that includes recommendations to support development and marketing of safe and effective artificial...
FDA clears AI-driven detection tool for pulmonary embolism
Medical imaging AI company Avicenna.AI has announced 510(k) clearance from the US Food and Drug Administration (FDA) for its CINA-iPE artificial intelligence (AI)-powered tool that...
US FDA seeks to “modernise” clinical trials with new draft guidance
The US Food and Drug Administration (FDA) has released draft guidance with updated recommendations for good clinical practices (GCPs) aimed at modernising the design...
Sky Medical Technology receives FDA clearance for (W3) geko device
Sky Medical Technology has announced it has achieved further US Food and Drug Administration (FDA) 510(k) clearance to market the new (W3) geko device...
FDA issues two final guidances for including patient perspectives in medical...
The US Food and Drug Administration (FDA) has issued two final guidances providing recommendations for including patient perspectives in medical device clinical studies.
As...
NIH grant sets Mercator research underway for local anti-inflammatory DVT therapy
Mercator MedSystems has recently announced that the DEXTERITY trials research has begun under a technology transfer grant for approximately $300,000 funded by the National...
Sky Medical receives FDA clearance to market the Geko device for...
Sky Medical Technology recently announced US Food and Drug Administration (FDA) 510(k) clearance to market the Geko device for increasing microcirculatory blood flow in...
FDA grants Breakthrough Device designation status for Hancock Jaffe’s VenoValve
Hancock Jaffe Laboratories today announced that the US Food and Drug Administration (FDA) has granted Breakthrough Device designation status to the VenoValve, the company’s...
Fist Assist device for focal arm massage and increased vein circulation...
Fist Assist Devices has received US Food and Drug Administration (FDA) 510(k) clearance for use of the Fist Assist FA-1 device in the USA...
Vesper DUO Venous Stent System may remove the need to “mix...
Mahmood Razavi (Orange, USA) speaks to Venous News about the recently initiated VIVID trial – a prospective, multicentre, single-arm study which has just begun enrolment and is designed...
Vesper Medical announces first enrolment in the VIVID trial
Vesper Medical recently announced initiation of its US Food and Drug Administration (FDA) investigational device exemption (IDE) study—VIVID (Venous stent for the iliofemoral vein investigational...
VIVO 12-month results show “very good early success, excellent safety and...
Anthony Comerota (Alexandria, USA), global principal investigator of the VIVO trial, discusses the highlights of the 12-month results of the VIVO clinical study that...
CDRH adjusts working practices with medical device companies to deal with...
The US Food and Drug Administration’s (FDA) Center for Devices and Radiological Health (CDRH) has written to the medical device industry to outline its...
Penumbra wins FDA clearance for Indigo Aspiration System
Penumbra has announced US Food and Drug Administration (FDA) 510(k) clearance for expanded indication of the Indigo Aspiration System. As part of the system,...
FDA approves first generics of Eliquis for prophylaxis of DVT and...
The US Food and Drug Administration (FDA) has announced the approval of two applications for the first generics of Eliquis (apixaban) tablets, to reduce...
FDA approval granted to calf stimulation device for patients at high...
UK-based Sky Medical Technology has received US Food and Drug Administration (FDA) 510(k) clearance for its "geko" device for stimulation of the calf muscles...
The new Venovo Venous Stent is changing clinical practice
Steven Dubenec (Sydney, Australia) and Gerard O’Sullivan (Galway, Ireland) discuss the impact of dedicated venous stents, in particular the Venovo venous stent (BD, formerly...
Scott Gottlieb resigns as head of US FDA
The commissioner of the US Food and Drug Administration (FDA), Scott Gottlieb, has unexpectedly resigned after serving just shy of two years in the...
US government shutdown disrupting FDA work
The US government shutdown means the country’s Food and Drug Administration (FDA) cannot accept new user fees, which means the agency cannot accept new...
US FDA plan shakeup of its 510(k) clearance programme
The US FDA has announced plans to modernise its 510(k) clearance programme for approving medical devices for the US market. Data show that about...
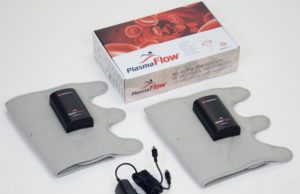
ManaMed launches PlasmaFlow digital and tubeless DVT prevention device
ManaMed has introduced PlasmaFlow, the first US Food and Drug Administrator-approved portable and tubeless deep vein thrombosis (DVT) prevention device throughout the USA.
"We at...
FDA grants priority review of Xarelto for a 10mg dose to...
The US Food and Drug Administration (FDA) has accepted, for priority review, a supplemental new drug application (sNDA) for Janssen’s Xarelto (rivaroxaban), to include...
FlowAid receives FDA US market clearance for the FA100 SCCD
Having received CE mark approval in July and Health Canada approval in August of last year, FlowAid Medical Technologies has now received US Food and Drug Administration (FDA)...
US FDA bans powdered gloves
The US Food and Drug Administration (FDA) has issued a final rule to ban powdered surgical gloves, patient examination gloves and the absorbable powder...
NICE collaborates with US FDA on Payer Communication Taskforce
The UK’s National Institute for Health and Care Excellence (NICE) has announced its participation in the US Food and Drug Administration (FDA)’s Payer Communication...
US FDA releases draft guidance on real-world evidence and medical device...
The US Food and Drug Administration (FDA) has released a draft guidance document entitled “Use of Real-World Evidence to Support Regulatory Decision-Making for Medical...